Chest Imaging And Bronchoscopy
Standard (two-dimensional) chest
X-rays to detect, diagnose or
follow morphological abnormalities in the chest are the mainstay of thoracic
radiographical imaging and account for more than 50% of procedures. Recent
innovations include digital, three-dimensional computed tomography (CT) scans
and physiological (positron emission tomography, ventilation-perfusion scans)
imaging. Specifi radiographical abnormalities are discussed in individual
chapters.
Posteroanterior (PA) and lateral chest
radiographs (CXRs) allow two-dimensional visualization of the lungs, great
vessels, heart, diaphragm and mediastinum. PA film should be performed upright
in full inspiration. Routine lateral film are not required for screening
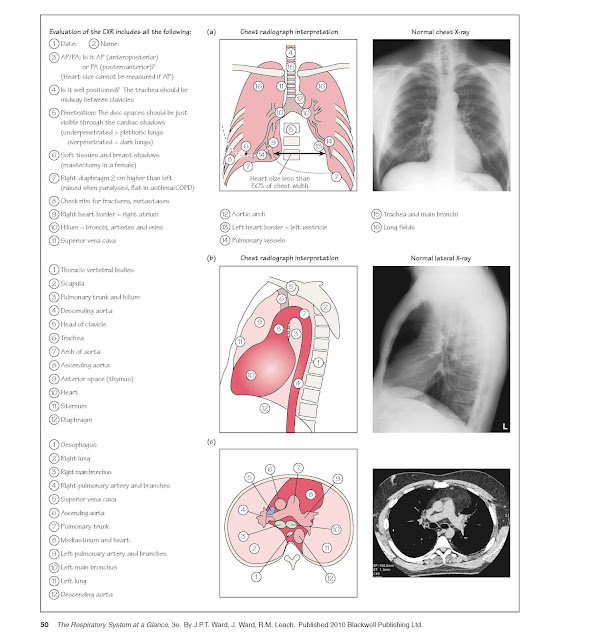
purposes. Figure 21a and 21b illustrates CXR features and interpretation.
Portable anterior-posterior film (AP) in patients unable to stand magnify the
heart and mediastinum and do not allow detailed visualization of lung
parenchyma.
A standard PA and lateral CXR should
allow visualization of both lungs, including the diaphragmatic position, as
well as the normal trachea, main carina, main stem bronchi, major and minor
fissures aorta, main pulmonary arteries and heart. Understanding of the normal
anatomy of a CXR is essential to allow recognition of abnormal lung parenchymal
infiltrates enlarged lymph nodes adjacent to the trachea or in the hila,
enlarged pulmonary arteries, volume loss of a lobe or segment or cardiac
enlargement. In the case of a suspected pleural effusion, lateral decubitus films allow visualization of as little as 50 mL of free-flowing fluid Digital
CXRs are being developed that allow more detailed views of the denser portions
of the thorax and show finer detail of the lung parenchyma.
|
.
|
|
.
|
|
.
|
|
.
|
|
.
|
Pulmonary emboli (PE): administration of intravenous contrast allows
imaging of the pulmonary blood vessels and detection of emboli. Examples of CT
scans are shown in several chapters. Newer technology allows complete axial
scanning of the thorax with a single breath-hold.
Ventilation–perfusion (V/Q) scans are mostly performed in the evaluation of
pulmonary embolism (Chapter 28). Gamma cameras can visualize
radiopharmaceuticals either injected into the venous blood (perfusion) or
inhaled (ventilation). Thromboembolism classically causes a V/Q mismatch,
with absence of perfusion in the presence of ventilation. Unfortunately, the
value of V/Q scans is limited by the observation that many PEs result in
indeterminate V/Q scans that show small mismatches or matched V/Q deficits In
these cases, other studies must be utilized to demonstrate thromboemboli.
Contrast CT scans are increasingly used to detect PE (see above) and are being
investigated as possible replacements for V/Q scanning. Quantitative V/Q scans
may be used in preparation for lung resection surgery, to assess regional lung
function and estimate the amount of residual lung function.
Pulmonary angiography visualizes the vasculature following injection of
contrast medium (Chapter 28). It may be required in patients with suspected
pulmonary emboli but equivocal V/Q scans, pulmonary hypertension and pulmonary
vascular disease, including vasculitis and arteriovenous malformations. These
studies are often preceded by echocardiography to visualize right ventricular
function and estimate pulmonary artery pressure using Doppler imaging.
Positron emission tomography (PET) utilizes a f uorinated analogue of glucose (FDG)
to give images of the lung that highlight areas of increased glucose
metabolism. Malignant cells have increased glucose uptake and appear as
increased densities on PET images. Recent studies have demonstrated that PET is
useful in distinguishing between benign and malignant solitary pulmonary
nodules and in detecting small nodal metastases that are not detected on CT
scanning. For these indications, PET has a sensitivity and specificit of 80-97%
with false-positive scans seen in cases of infection or granulomatous
inflammation Whole body PET was recently used to detect clinically inapparent
distant metastases.
Bronchoscopy enables direct visualization down to the fourth
and fifth divisions of the endobronchial tree. Chest physicians perform most
bronchoscopies as day cases under local anaesthetic in the sedated but awake
patient, using a flexible fibreopti instrument. It has the advantages of
visualization of the upper lobes and is a safe technique with a low
complication rate. Saturation and heart rhythm should be monitored and
supplemental oxygen should be administered during the procedure. Facilities for
resuscitation should always be immediately available. Thoracic surgeons may use
a rigid bronchoscope in the fully anaesthetized patient. This instrument allows
larger biopsies and better suctioning, and is the method of choice when
removing inhaled foreign bodies. Bronchoscopy is most frequently performed to
investigate if a shadow on a chest radiograph is due to a lung cancer (Chapter
40). If an endobronchial tumour is seen, biopsies for histological analysis and
washings and brush samples for cytological analysis can be taken. In addition,
information regarding the operability of the tumour can be obtained.
Bronchoscopy can also be used to diagnose parenchymal lung disease using the
technique of transbronchial biopsy, which obtains parenchymal and bronchial
tissue for histological examination. Collection of bronchoalveolar fluid
(bronchoalveolar lavage, BAL) is useful in diagnosing alveolitis (raised lymphocyte
count in sarcoidosis), infection in the immunocompromised patient (e.g. Pneumocystis
carinii pneumonia) and tuberculosis. Bronchoscopy also aids investigation
of collapsed segments or lobes. Therapeutically, bronchoscopy is used to remove
inhaled foreign bodies, to aspirate sputum plugs and secretions, to relieve
stenosis by placement of stents and during treatment of endobronchial tumours
with laser or endobronchial radiotherapy. Haemorrhage, pneumothorax and cardiac
arrhythmia, although uncommon, are the main complications of
fibreoptinchoscopy.