Skin Physiology : The Process Of
Keratinization
Keratinization, also known as cornification, is unique to the epithelium
of the skin. Keratinization of the human skin is of paramount importance; it
allows humans to live on dry land. The process of keratinization begins in the
basal layer of the epidermis and continues upward until full keratinization has
occurred in the stratum corneum. The function and purpose of keratinization is
to form the stratum corneum.
The stratum corneum is a highly organized layer that is relatively strong
and resistant to physical and chemical insults. This layer is critically important
in keeping out microorganisms; it is the first line of defense against
ultraviolet radiation; and it contains many enzymes that can degrade and
detoxify external chemicals. The stratum corneum is also a semipermeable
structure that selectively allows different hydrophilic and lipophilic agents
passage. However, the most obvious and most studied aspect of the stratum
corneum is its ability to protect against excessive water and electrolyte loss.
It acts as a barrier to keep chemicals out, but more importantly, it keeps
water and electrolytes inside the human body. Transepidermal water loss (TEWL)
increases as the stratum corneum is damaged or disrupted. The main lipids
responsible for protection against water loss are the ceramides and the
sphingolipids. These molecules are capable of binding many water molecules.
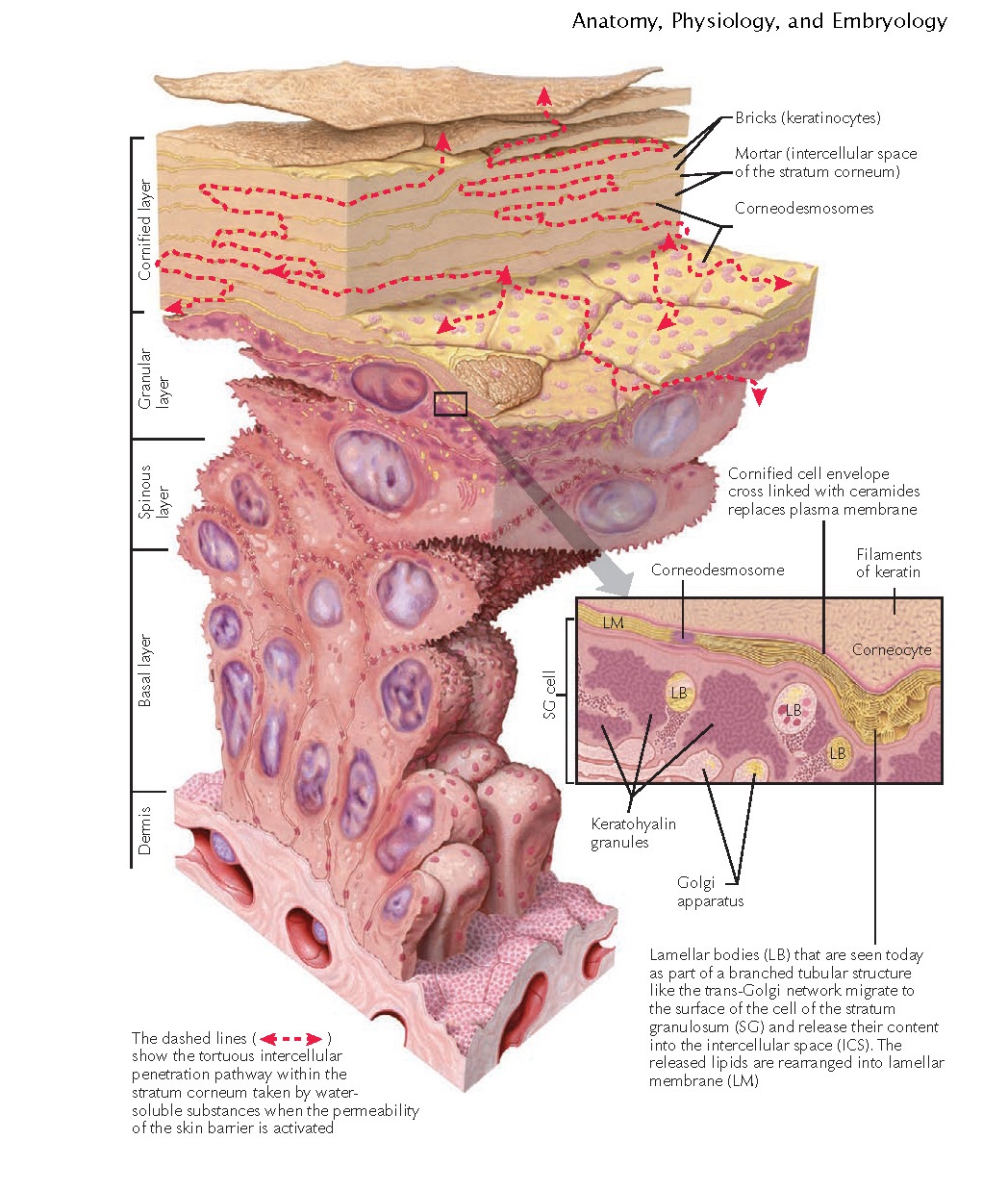
As keratinocytes migrate from the stratum basale and journey through the
layers of the epidermis, they undergo characteristic morphological and
biochemical changes. The keratinocytes flatten and become more compacted and
polyhedral. The resulting corneocytes become stacked, like bricks in a wall.
These corneocytes are still bonded together by desmosomes, which are now called
corneodesmosomes.
The stratum granulosum gets its name from the appearance of multiple
basophilic keratohyalin granules present within the keratinocytes. These
granules are largely composed of the protein profilaggrin. Profilaggrin is
converted into filaggrin by an intercellular endo- proteinase enzyme. Filaggrin
is so named because it is a filament-aggregating protein. Over time, filaggrin
is broken down into natural moisturizing factor (NMF) and urocanic acid. NMF is
a breakdown product of filaggrin that slows water evaporation from the
corneocytes.
The intercellular space is composed of lipids and water. The lipids are
derived from the release of the lamellar bodies (Odland bodies). Ceramides make
up the overwhelming majority of the contents of the lamellar bodies. Other
components include free fatty acids, cholesterol esters, and proteases. The
lamellar bodies fuse with the cell surface and release their contents into the
intercellular space. The fusion of the lamellar body with the cell surface is
dependent on the enzyme trans-glutaminase I.
Concurrently. the cornified cell envelope (CCE) develops. The CCE proteins
envoplakin, loricrin, periplakin, small prolinerich proteins, and involucrin
are cross-linked in various arrangements by transglutaminase I and
transglutaminase III, forming a sturdy scaffolding along the inner surface of
the keratinocyte cell membrane. As
the keratinocyte migrates upward, the cell membrane is lost, and the ceramides
that are released begin cross-linking with the CCE proteins. The cells continue
to move toward the surface of the skin and begin to lose their nucleus and
cellular organelles. The loss of these organelles is mediated by the activation
of certain proteases that can quickly degrade protein, DNA, RNA, and the nuclear
membrane.
Once the cells reach the outer layers of the stratum corneum, they begin
to be shed. On average, a keratinocyte spends 2 weeks in the stratum corneum
before being shed from the skin surface in a process called desquamation. Shedding is achieved by the
final degradation of the corneodesmosomes by proteases that destroy the
desmoglein-1 protein.
Keratinization is especially important in the diseases of cornification.
Many skin diseases have been found to involve defects in one or more proteins
that are critical in the process of cornification. Examples are lamellar
ichthyosis, which is caused by a defect in the transglutaminase I enzyme, and
Vohwinkel’s syndrome (keratoma hereditarium mutilans), which results from a
genetic mutatio in the loricrin protein and a resultant defective CCE.