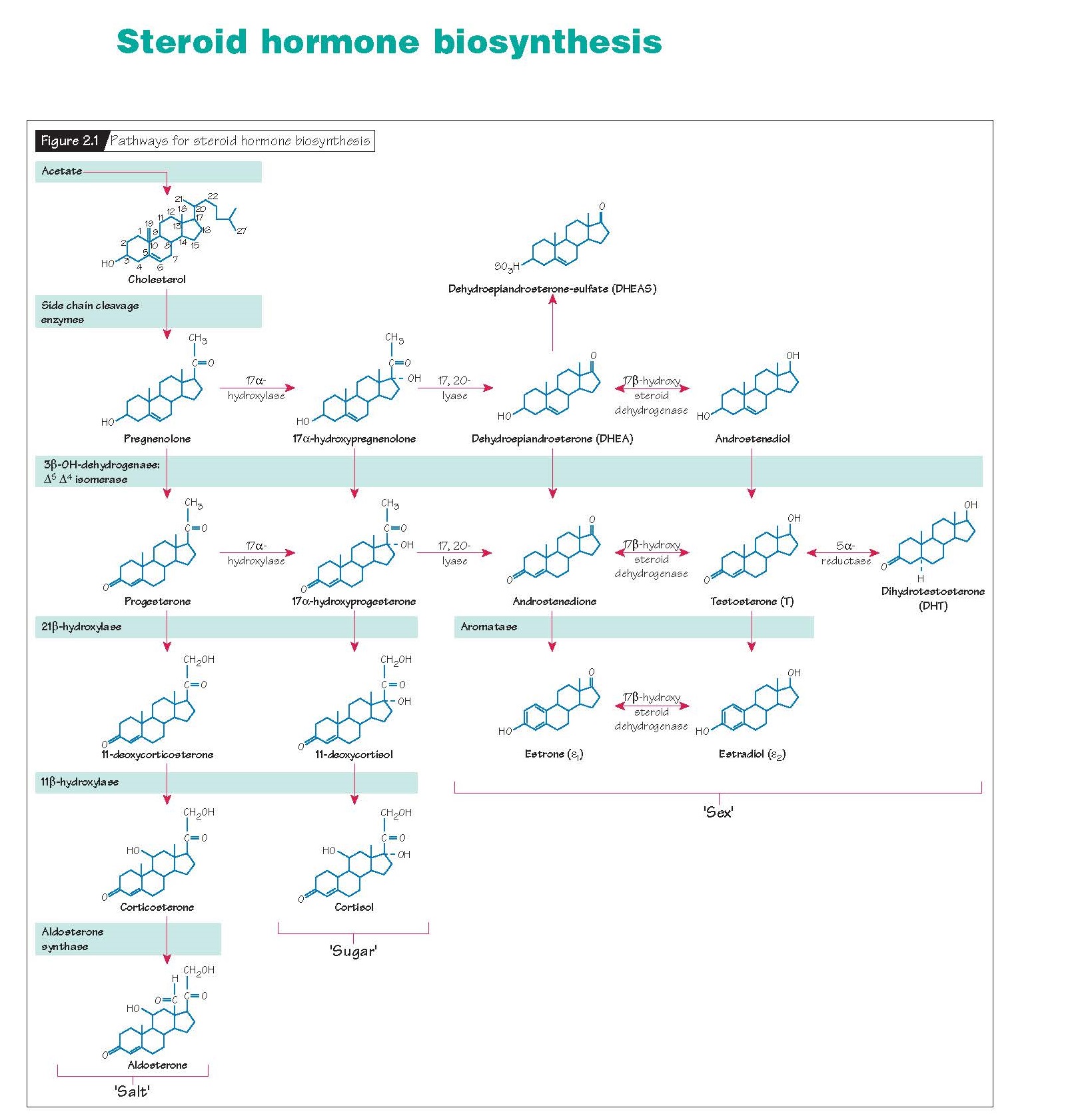
Steroid Hormone Biosynthesis.
Cholesterol and the steroid production pathway Cholesterol is the building block of
steroid hormones. All steroid-producing organs with the exception of the
placenta can synthesize cholesterol from acetate. Under most circumstances,
however, local synthesis cannot meet demand and circulating cholesterol must be
used. The major carriers of cholesterol in the bloodstream are the low density
lipoproteins (LDLs). LDL is removed from the blood by steroidogenic cells
using cell surface receptors that recognize specific surface proteins on LDL
called apoproteins. Once in the cell, cholesterol is carried through a sequence
of enzymatic changes to produce a final product that belongs to one of the
major classes of steroid hormones: progestins, androgens and estrogens (sex),
glucocorticoids (sugar) and mineralocorticoids (salt). All steroid-producing
tissues use a common sequence of precursor molecules and enzymes (Fig.
2.1). Tissue specificity is conferred by the presence or absence of specific
enzymes in the sequence. For instance, the gonads differ from the adrenal
glands in that ovaries and testes do not express the 21-hydroxylase or
11β-hydroxylase enzymes that are necessary to produce corticosteroids.
Therefore, the gonads only produce three classes of steroids: progestins,
androgens and estrogens.
During conversion of cholesterol to steroid metabolites, the number of
total carbon atoms decreases sequentially. Progestins have 21 carbons (C-21);
androgens have 19 carbons (C-19); and estrogens have 18 carbons (C-18). Thus,
progestins are obligatory precursors of both androgens and estrogens. Likewise,
androgens are obligatory precursors of estrogens.
Most of the steroidogenic enzymes are members of the cytochrome P450
class of oxidases. A single mitochondrial protein P450scc, the cholesterol
side chain cleavage enzyme, mediates all steps in the con- version of
cholesterol to pregnenolone. The activity of this protein represents the
rate-limiting step for the entire steroid pathway. Not surprisingly, it is also
the major site of tropic hormone stimulation. Genetic mutations of P450scc are
very rare and usually lethal. No steroid hormones can be produced by an
individual with an inactive P450scc enzyme.
Once pregnenolone is formed, steroid production can proceed down one of
two paths, through either progesterone or 17α- hydroxypregnenolone. All but two
of the enzymes responsible for producing the steroid hormones are packaged
within the endoplasmic reticulum, together with other members of the P450
system. The bio-synthetic units are very tightly linked together, thereby
ensuring that very few of the steroid intermediates leave the cell. This
packaging is also highly efficient in that it can convert an entire class of
steroids to another. Thus, 17,20-desmolase will convert all progestins to androgens,
and aromatase will convert all androgens but dihydrotestosterone (DHT) to
estrogens.
Sites of production
Ovary
In the ovary, steroid production occurs in a two-cell system (Fig.
2.2). Theca cells produce androgens. These androgens diffuse into the granulosa
cells where they are converted to estrogens. Tropic hormones regulate specific
steps in the sex steroid cascade. Theca cells respond to luteinizing hormone
(LH) by increasing the number of LDL receptors and hence cholesterol entry
into the cells. LH also stimulates P450scc activity,
allowing increased androgen production. When these androgens diffuse into
granulosa cells, they are metabolized by aromatase into estrogens. Follicle-stimulating
hormone (FSH) induces the activ- ity of aromatase in the granulosa cell,
thereby increasing the conversion of androgens to estrogens. The single
aromatase gene has many promoter sites. These are responsive to cytokines,
cyclic nucleotides, gonadotropins, glucocorticoids and growth factors.
Testes
In the testes, androgen production occurs largely in the Leydig cells
under the influence of LH. Androgens produced in the Leydig cells either enter
the bloodstream directly or diffuse into nearby Sertoli cells. Sertoli cells
can convert androgens to estrogens using aromatase or reduce them to
dihydrotestosterone via 5α-reductase. Because the specific cell types within
the differentiated male and female gonads have common embryonic origins
(Chapter 5), the mechanisms for steroid production in the testes very much
parallel those in the ovary.
Adrenals
Sex hormone production by the adrenals occurs largely as a by-product of
corticosteroid biosynthesis. The contribution of adrenal sex steroids to the
total pool of circulating sex steroids is typically small, although there are
several important exceptions. The ovaries of postmenopausal women no longer
produce significant amounts of steroid hormones so adrenal androgen production
can become clinically significant. In pregnancy, the placenta cannot synthesize
cholesterol from acetate. Rather, it relies on adrenal androgens of both
maternal and fetal origin to make estrogens.
With the exception of P450scc, inherited defects in any of the enzymes
involved in steroidogenesis are associated with clinical syndromes resulting
either from accumulation of a precursor product or absence of a key
end-product. For example, an inherited deficiency of the enzyme 21-hydroxylase
in the adrenal gland will lead to a deficiency in adrenal cortisol production.
Low cortisol levels feedback to promote enhanced production of adrenal
glucocorticoids. The enzymatic block, however, results in an accumulation of
precursor progestins, some of which will be shunted down functional androgen
biosynthetic pathways. If the 21-hydroxylase deficiency occurs in a female
fetus, the increase in androgen production may cause masculinization of the
external genitalia, known as congenital adrenal hyperplasia syndrome (Chapter
27). Similarly, disorders of male sexual differentiation and development may
result from genetic defects in androgen
production (Chapter 26).