Organ
Preservation
Cellular integrity depends on the function of membrane pumps, which
maintain the intracellular ion composition. These pumps use high-energy
phosphate molecules such as adenosine triphosphate (ATP) as their energy
source. ATP is generated from ADP via a series of chemical reactions, which
require sugars, amino acids or fatty acids as substrate. Aerobic metabolism is
19 times more efficient than anaerobic metabolism in generating ATP. ATP and other high-energy phosphate molecules
are also important for other metabolic processes within a cell.
When the circulation to an organ stops, it switches from aerobic to
anaerobic metabolism. Since there is no substrate reaching the cells from which
ATP can be generated, cellular ATP stores rapidly deplete, membrane pumps fail
and cellular integrity is lost. Other energy-dependent metabolic pathways also fail.
Principles of organ preservation
Organ preservation aims to reduce the effects of ischaemic injury by a
combination of cooling and use of special preservation solutions.
Cooling
Cooling an organ by 10°C halves the metabolic rate, and cooling to 4°C
reduces metabolism to less than a tenth of the rate at normal body temperature.
There are two ways to cool an organ, core-cooling and topical cooling. Core
cooling involves flushing the organ with ice-cold preservation solution via its
arterial supply. It is rapid and effective, but a large volume of fluid is
needed to cool an organ quickly, since heat transfer is slow. Topical cooling
involves immersing an organ in saline ice slush, or placing slush topically
over the organ in the deceased donor while organ removal proceeds. Topical
cooling is very inefficient compared with core cooling, and it really only
works well in small children or for small organs with large surface area to
volume ratio, such as the pancreas. In reality, a combination of core cooling
and topical cooling are employed.
Preservation solutions
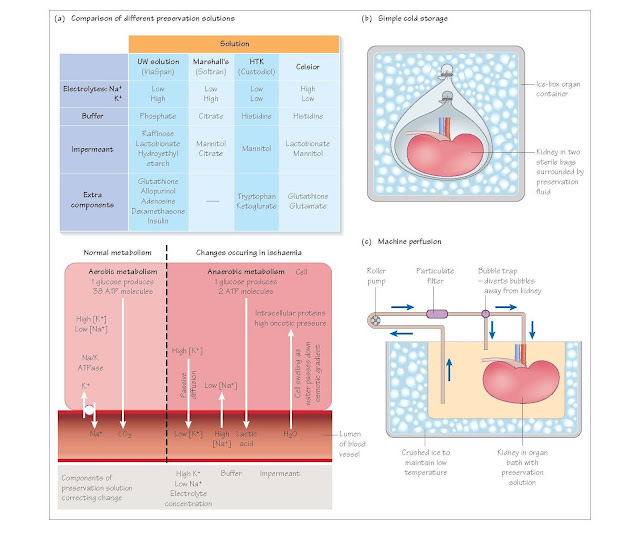
Organ preservation solutions aim to minimise the cellular changes
occurring during cold storage. They comprise three principal components.
Electrolytes
The intracellular electrolyte composition is characterised by high
potassium and low sodium concentrations, in contrast to the low potassium, high
sodium milieu that surrounds the cells. Early preservation solutions used an
electrolyte composition more akin to intracellular fluid to minimise the
diffusion that occurs in the cold when the Na/K ATPase pumps fail. In fact,
there appears to be no benefit in having an intracellular composition, and
indeed a high potassium concentration in the preservation fluid causes
vasospasm and may cause problems on reperfusion, particularly of the liver,
when the preservation fluid is washed out of the organ into the circulation (it
may induce ventricular arrhythmias).
Impermeants
Impermeants are osmotically active substances such as lactobionate and
raffinose, which stay outside the cells and so prevent cell swelling by
countering the osmotic potential of the intracellular proteins. Some solutions,
such as UW solution, also contain a colloid component (hydroxyethyl starch).
Buffer
Anaerobic metabolism results in the accumulation of metabolites,
including lactic acid. To keep the extracellular milieu at a fixed pH, the preservation
solutions contain a buffer. The nature of the buffer varies between the
different solutions.
Additional reagents
Some solutions have additional compounds that may add substrate for
metabolism, scavenge harmful metabolic products, and so on.
Preservation solutions in practice
Traditionally used solutions for abdominal organs include Ross and
Marshall’s hypertonic citrate solution for kidneys and Belzer’s University of
Wisconsin (UW) solution for liver, kidney and pancreas; more recently other solutions
such as Bretschneider’s histi- dine-tryptophan-ketoglutarate (HTK) solution and
Celsior have been developed as multi-organ preservation solutions. Using these
solutions it is possible to keep a liver or pancreas for 18 hours and a kidney
for 36 hours, although the shorter the cold ischaemic period the better
(typically less than 11 hours for liver and pancreas, and less than 18 hours
for a kidney).
Preservation of the heart uses high-potassium cardioplegia solutions to
stop the heart, but tolerance to cold ischaemia using these electrolyte
solutions is poor and cold storage of the heart beyond 4 hours is undesirable.
Preservation of the lungs is different again, and there is no clear
consensus on the best perfusion fluid, though solutions with an extracellular
ion composition seem to be better than the more traditional ‘intracellular’
fluids. Initial ischaemic injury to the lungs can be ameliorated by
insufflating them with oxygen, some- thing that has greatest benefits in lungs
donated after circulatory death.
Static storage or machine perfusion
Static cold storage
The simplest method of preservation is to flush cold preservation
solution through an organ, and then store the organ in preservation solution in
an ice-box. It has the advantage of low cost and simplicity.
Continuous cold perfusion
An alternative for kidneys, this involves connecting the kidney to a
machine that pumps ice-cold preservation solution through the artery in a
circuit, thus removing waste products and providing new energy substrates. This
is probably superior to static cold storage for long preservation periods, but
is more costly and offers little benefit for short durations of ischaemia.
Normothermic perfusion
There has been much recent interest in creating an artificial circulation
to pump oxygenated blood through an organ to keep it functioning as normal, so
avoiding ischaemia. Prototypes exist for all the thoracic and abdominal organs currently transplanted.