Innate
Immunity
The role of the immune system is to identify and remove invading
microorganisms before they cause harm to the host. This is achieved by a rapid,
non-specific innate immune response that is followed by a more finely tuned,
targeted, adaptive immune response. The innate immune system is comprised of components
that directly recognise and destroy pathogens (the complement system), a number
of ‘flags’ known as opsonins (e.g. C-reactive protein [CRP], C3b, natural IgM
antibody), which make pathogens more easily recognised by immune cells such as
phagocytes (neutrophils and macrophages), which engulf and kill
internalised pathogens, and natural killer (NK) cells, which can detect and
destroy virus-infected cells.
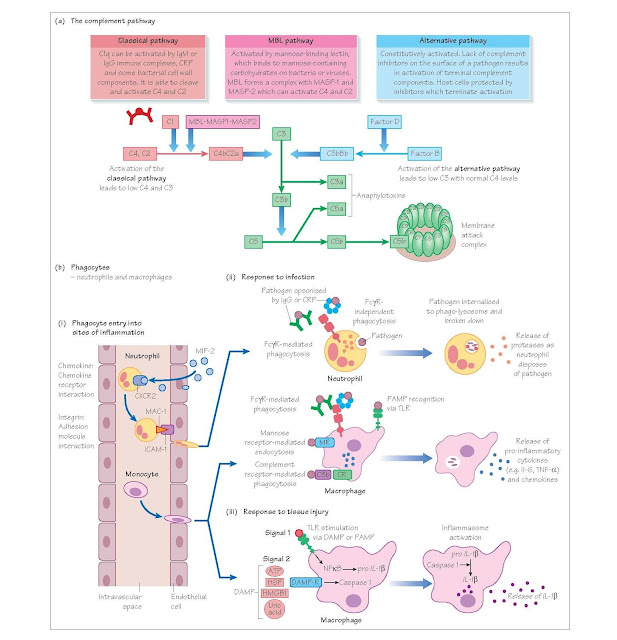
The complement system
The complement system is a series of proteases, which are sequentially
activated and culminate in the formation of the membrane attack complex (MAC).
The MAC forms a hole in the membrane of the cell into which it is inserted
(pathogen or host), disrupting membrane integrity and causing cell lysis. The
complement system can be activated in three ways:
1
the classical pathway
2
the alternative pathway
3
the mannose binding pathway.
IgM or immune complexed IgG activate the classical pathway. The
alternative pathway is constitutively active, while the mannose binding pathway
is activated by carbohydrates present on pathogens. The net result of
activating any of the three pathways is the formation of a C3 convertase
(either C4bC2a or C3bBb), which cleaves C3. The resulting C3b cleaves C5 and
activates a final common pathway resulting in MAC formation. Complement activation
also leads to the production of anaphylotoxins (C3a and C5a), which activate
neutrophils and mast cells, promoting inflammation. In addition, C3b can
opsonise pathogens for uptake by complement receptors CR1 and CR3 on
phagocytes.
Pentraxins
These are a family of proteins with a pentameric structure that include
CRP and serum amyloid protein (SAP). CRP and SAP are synthesised in the liver
and rapidly released into the bloodstream in response to inflammation and are
therefore called acute phase proteins. Pentraxins bind to phosphorylcholine
found on the surface of pathogens and can fix complement (via the classical
pathway) and opsonise pathogens for uptake by phagocytes through binding to
surface Fc-gamma receptors (FcγRs). Pentraxins can also bind to apoptotic
cells, facilitating their disposal.
Phagocytes
Phagocytes (from the Greek word ‘phagein’ – ‘to eat’) are cells that
ingest debris, pathogens and dying cells. There are two main types of
phagocyte, neutrophils (which circulate in the blood until they are called into
tissues), and macrophages, which are resident in tissues and act as immune
sentinels. The circulating monocyte is the precursor to tissue macrophages.
Neutrophils are the most abundant circulating leucocyte and can be identified by their multi-lobed
nucleus and the presence of numerous granules within their cytoplasm, which
contain proteases (for example myeloperoxidase) and other bacteriocidal
substances. Neutrophils move into tissues by virtue of surface molecules called
integrins (for example MAC-1), which bind to adhesion molecules that are upregulated
on vascular endothelium in inflamed tissue (for example ICAM-1).
Phagocytes detect pathogens via membrane receptors, which recognise
repeating surface motifs on microbes, so-called pathogen-associated molecular
patterns (PAMPs). These innate receptors include the toll-like receptors (TLRs)
and the mannose receptors. Phagocytes can also internalise opsonised pathogens
via complement receptors and FcγRs. Once internalised, the microbe will be
destroyed within the phagolysosome by proteases and by the generation of oxygen
and nitrogen free radicals. Tissue- resident macrophages secrete
pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α and
interleukin (IL)-6, which lead to changes in vascular permeability, and in the
molecules expressed on vascular endothelial cells. They also produce chemicals
that attract neutrophils and monocytes (known as chemokines). These changes
facilitate the entry of neutrophils and monocytes from the circulation into the
site of infection and result in the cardinal signs of inflammation (calor,
dolor, rubor and tumor, i.e. heat, pain, redness and swelling).
Macrophages can also be activated by danger/damage- associated molecular
patterns (DAMPs), for example heat shock proteins (HSPs) or ATP, which are
release by damaged or dying host cells. This leads to activation of the
inflammasome and the production of IL1-β and IL18. In addition, macrophages have the capacity to process and present antigen (see Chapter 8).
Mast cells
Mast cells are large tissue-resident cells found mainly in the skin and
at mucosal surfaces. They are packed with granules containing vasoactive amines
(e.g. histamine) and heparin. Mast cell degranulation may be induced by trauma
or UV light, and by binding of IgE antibodies to Fc-epsilon receptors found on
the surface of mast cells. Mast cells play an important role in allergy and
anaphylaxis.
Natural killer cells
Natural killer cells express surface receptors (killer-cell immunoglobulin-like
receptors [KIRs]), which bind to and assess cell surface major histocompatibility
complex (MHC) class I molecules. If non-self or altered self-antigen is
detected on class I molecules, e.g. in virally infected cells or tumour cells,
then the NK cell will destroy this cell by the release of perforin (punches
holes in cells), granzyme (poisons cells) or the induction of apoptosis. In
addition, NK cells express FcγRs and can therefore be activated against
antibody-opsonised cells. This is known as antibody-dependent cellular
cytotoxicity (ADCC).