Diffusion
Oxygen and carbon dioxide are transported in the body by a mixture of bulk
flow and diffusion. Bulk flow, generated by differences in total fluid pressure, is important in most of
the airways and in transporting blood containing these gases between pulmonary
and tissue capillaries. Diffusion, driven by partial pressure differences, is
important in the last few millimetres of the airways, across the
alveolar-capillary membrane and between tissue capillaries and mitochondria.
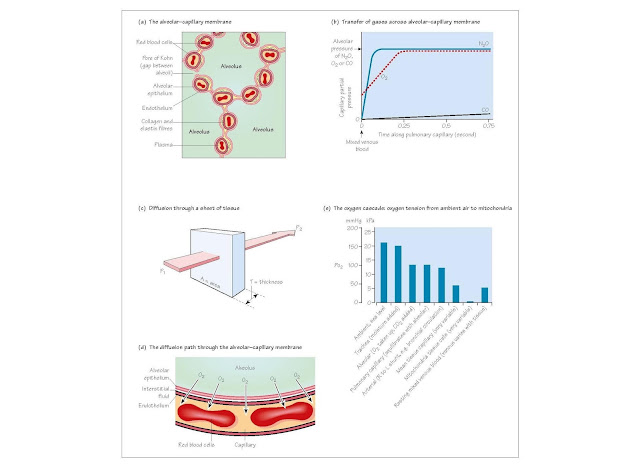
The alveolar–capillary membrane (Fig. 5a) Adult male lungs contain
about 300 million alveoli, approximately 0.2
mm in diameter. Between neigh bouring
alveoli are two layers of alveolar epithelium each resting on a basement
membrane, enclosing the interstitial space, containing pulmonary capillaries,
elastin and collagen fibres. The alveolar epithelium and capillary
endothelium form the alveolar–capillary membrane, through which
gases diffuse. It is very thin (<0.4 μm), except where collagen and
elastin fibre are concentrated, with a total surface area of about 85 m2. There
are two types of alveolar epithelial cells. Type I pneumocytes line the
alveoli and are relatively devoid of organelles. The round type II
pneumocytes have large nuclei, microvilli and contain striated osmiophilic
lamellar bodies storing surfactant, an important component of alveolar lining fluid (Chapter 6).
Diffusion and perfusion limitation (Fig. 5b)
If gas containing the poorly soluble gas nitrous oxide (N2O)
is inhaled, pulmonary capillary Pn2o rises and quickly
equilibrates with alveolar Pn2o. With no alveolar-capillary
partial pressure gradient remaining, diffusion ceases along the rest of the
pulmonary capillary and uptake can only be increased by increasing pulmonary
capillary blood flow. N2O uptake is said to be perfusion-limited.
In contrast, when breathing a carbon monoxide (CO) containing mixture, the CO
combines so avidly with haemoglobin that pulmonary capillary Pco rises
little. The pressure gradient driving diffusion is preserved along the capillary,
and CO uptake would not be increased by increased perfusion. Improved ease of
diffusion, with reduced thickness or increased area of the alveolar-capillary
membrane, would increase CO uptake. CO transfer is diffusion-limited.
Oxygen transfer lies between these two extremes, but is normally
perfusion-limited.
Factors affecting diffusion across a membrane (Fick and Graham’s laws)
For a sheet of tissue of area A and thickness T through which gas g is
passing (Fig. 5c):
A Rate of transfer
of gas, g ∝  ( P1 – P2 )
( P1 – P2 )
The constant of proportionality
Although the molecular weight of CO2 is about 1.4 times that
of O2, it is about 20 times more soluble, and so diffuses more
easily.
For the alveolar-capillary membrane, the pressure gradient driving
diffusion is alveolar (PA) minus mean pulmonary capillary (PC-).
The constants (s, mw, A and T) can be combined to give a single constant, the diffusing
capacity (DLg) of the lungs for gas, g:
Rate
of transfer of gas, g = DLg( PA − PC-)
Oxygen diffusing capacity, DLo2
= Oxygen uptake from the lungs (Y- o2
)
PAo2 − PC-o2
Although measurement of DLo2 is desirable,
it is not possible because mean
capillary Po2 (
PC-o2)
cannot be measured.
CO diffuses through the same pathway as O2, and its rate of
diffusion is affected by the same factors that affect oxygen transfer. However,
unlike DLo2, DLco is measurable.
Once CO arrives in the pulmonary capillary blood, it too combines with
haemoglobin. Haemoglobin has approximately 240 times the affinity for CO than
it does for O2, and consequently as CO is transferred, almost all of
it enters chemical combination and the mean pulmonary capillary Pco can
be assumed to be zero.
This simplifie the equation to:
DLco = Carbon monoxide uptake from the lungs (Vco)
PAco
Several methods are used for measuring DLco, but all
involve breathing a low level of CO (e.g. 0.3%). By sampling exhaled gas, CO
uptake and mean alveolar Pco can be calculated. The normal value depends
on the method used, but is about 15-30 mL/min per mmHg (112-225 mL/min per
kPa). A tracer gas, such as helium, is included in the gas mixture so that
alveolar volume can also be measured (see Chapter 20). DLco
is divided by alveolar volume to give an index (Kco) that corrects for
different lung volumes. As both DLo2 and DLco
are affected by the rate of gas combination with haemoglobin in addition to
factors affecting diffusion, the alternative term, transfer factor (TLo2
and TLco), is more commonly used in Europe.
Factors affecting DLco (TLco)
DLco is lowered by reduced alveolar-capillary membrane
area in emphysema, pulmonary emboli or lung resection and by increased thick-
ness in pulmonary oedema. In pulmonary fibrosi the alveolar-capillary membrane
is both thickened and reduced in area giving a low DLco with
a low but less affected Kco. Increased pulmonary blood volume in
exercise increases the effective area increasing DLco. DLco
is increased with polycythaemia and reduced in anaemia. DLco
is therefore non-specific but it is sensitive and may reveal abnormalities when
other lung function tests are normal. Hypoventilation does not affect DLco
because the reduced CO uptake is caused by reduced PAco.
The oxygen cascade (Fig. 5e) shows how Po2 falls
between air and mitochondria. Mitochondrial oxidative phosphorylation will
cease when Po2 falls below 1 mmHg (0.13 kPa), and this
ultimately limits the capillary Po2 that can be tolerated and
therefore the amount of oxygen that can be removed as blood passes through the
tissues. Capillary Po2 must remain high enough to drive
diffusion to cells at a rate sufficien to match oxygen consumption and maintain
mitochondrial Po2 above ritical
level.