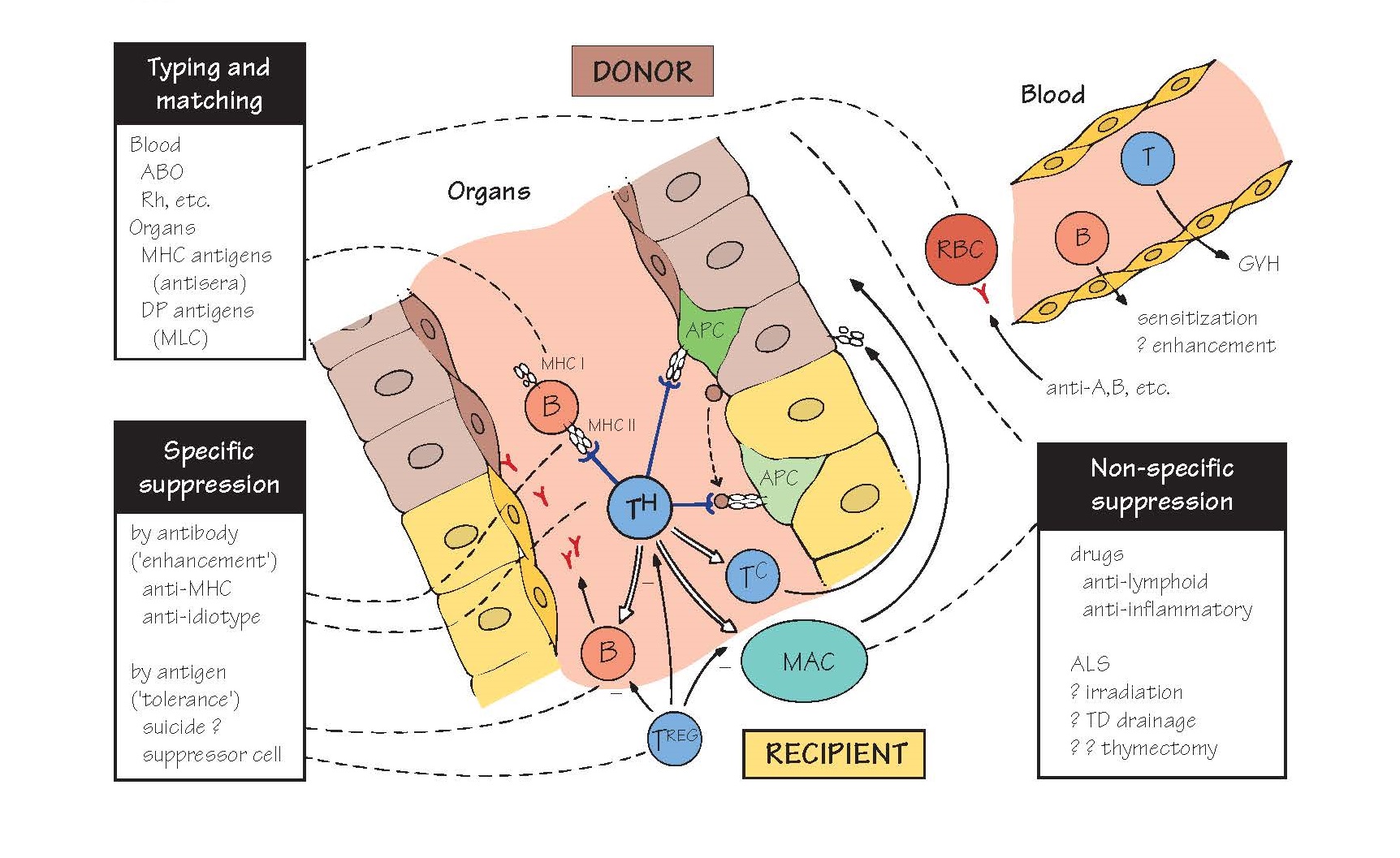
Transplant Rejection
The success of organ grafts between identical
(‘syngeneic’*) twins, and their
rejection in all other cases, reflects the remarkable strength of immunological
recognition of cell-surface antigens within a species. This is an unfortunate
(and in the evolutionary sense unforeseeable) result of the specialization of T
cells for detecting alterations of MHC antigens, upon which all adaptive
responses depend (for a reminder of the central role of T-helper cells see Figs
19 and 21), plus the enormous degree of MHC polymorphism (different
antigens in different individuals; see Fig. 11). It appears that when
confronted with ‘non- self’ MHC molecules, T cells confuse them with ‘self plus
antigen’, and in most cases probably ‘self plus virus’; several clear examples
of this have already been found in mouse experiments. This may be one of the
reasons for MHC polymorphism itself: the more different varieties of ‘self’ a
species contains, the less likely is any particular virus to pass undetected
and decimate the whole species. Differences in red cell (‘blood group’)
antigens also give trouble in blood transfusion (top right) because of
antibody; here the rationale for polymorphism is less obvious, but it is much
more restricted (e.g. six ABO phenotypes compared with over 1012 for MHC). The
‘minor’ histocompatibility and blood group antigens appear to be both less polymorphic
and antigenically weaker.
Graft rejection can be mediated by
T and/or B cells, with their usual non-specific effector adjuncts (complement,
cytotoxic cells, macrophages, etc.), depending on the target: antibody destroys
cells free in the blood, and reacts with vascular endothelium (e.g. of a
grafted organ; centre) to initiate type II or III hypersensitivity, while T
cells attack solid tissue directly or via macrophages (type IV). Unless the
recipient is already sensitized to donor antigens, these processes do not take
effect for a week or more, confirming that rejection is due to adaptive, not
innate, immunity.
Successful organ grafting relies at
present on (top left) matching donor and recipient MHC antigens as far as possible
(relatives and especially siblings are more likely to share these), and (bottom
right) suppressing the residual immune response. The ideal would be (bottom
left) to induce specific unresponsiveness to MHC antigens, but this is still experimental (see Fig. 40).
For blood transfusion, the
principle is simple: A and/or B antigens are detected by
agglutination with specific antisera; this is always necessary because normal
individuals have antibody against whichever antigen they lack. Rh (Rhesus)
antigens are also typed to avoid sensitizing women to those that a
prospective child might carry, as Rh incompatibility can cause serious
haemolytic disease in the fetus. Minor antigens only cause trouble in patients
sensitized by repeated transfusions. Other possible consequences of blood
transfusion are sensitization against MHC antigens carried on B cells
and, in severely immunodeficient patients, GVH (graft-versus-host)
reactions by transfused T cells against host antigens. The latter is a major
complication of bone-marrow grafting.
For organ (e.g. kidney) grafting,
MHC antigens must be typed by DNA typing, in which haplotype is determined
using polymerase chain reaction (PCR) and allele-specific pairs of primers. The
success of kidney grafting is related to the degree of match, particularly
class II (DR), although the better results with relatives suggest that there
are other ‘minor’ histocompatibility loci, which are still being identified.
The initial event is the recognition
of ‘altered self’ class II antigens by T-helper cells. This can occur either by
direct contact with donor B cells or antigen-presenting cells (light green APC
in figure) or via the uptake of soluble donor antigens (shaded circles) by the
recipient’s own APC (darker green). After this, B cells, cytotoxic T cells and
macrophages are all triggered into action, which response destroys the graft
depends on the organ in question. Some points of special interest are listed
below.
Kidney graft rejection can be immediate, due to
ABO mismatch or pre-existing anti HLA antibodies, acute (weeks to
months) due to the immune response or chronic (months to years) due to
repeated minor rejection episodes or re-emergence of immune complex-mediated disease.
Surprisingly, blood transfusion before grafting improves survival, perhaps by
inducing enhancing antibodies against class II donor antigens.
Immunosuppression has improved transplant success to over 70%, principally by
decreasing the occurrence of acute rejection. The causes of chronic rejection,
in contrast, remain poorly understood.
Bone marrow contains the haemopoietic stem cell, and is
therefore required whenever it is necessary to replace the host haemopoietic
system (e.g. in some immunodeficiencies or after high-dose chemo- therapy). The
growth factor G-CSF causes haemopoietic stem cells to come out of the bone
marrow and enter the circulation. As a result, blood can be used in place of
bone marrow, a procedure known as peripheral stem cell transplantation. Any
haemopoietic grafts are vigorously rejected, and require strong
immunosuppression. In addition, they can kill the host by GVH reaction, unless
T cells are removed from the donor marrow. In some cases GVH by the graft can
help to kill the original tumour cells (graft-versus-tumour [GVT]), but
balancing GVT and GVH remains a difficult clinical challenge.
Liver grafts are not so strongly rejected, and may
even induce a degree of tolerance. HLA typing is less important. Sometimes,
temporary organ transplants may be sufficient. In a recent example, a boy whose
liver was damaged by a virus infection received adult liver cells coated with a
chemical found in algae which prevented them from being attacked by the immune
system. The donor cells survived a few months, long enough for the recipient’s
liver to recover normal function.
Endocrine organs survive unexpectedly well if cultured or
otherwise treated to remove the minority of cells expressing class II antigens.
Skin grafts are rejected very vigorously by T cells,
perhaps because of their extensive vascularization. For this reason, skin
transplantation is usually autologous or a temporary graft is used to protect
the underlying tissue while the host’s own skin regenerates (e.g. after
extensive burns).
Cornea and cartilage, being non-vascular
tissues (immune privileged sites), are less accessible to the immune system.
Nevertheless, corneas are rejected in about 25% of cases, although the
mechanisms leading to graft recognition remain unclear.
The normal fetus is of
course an allograft, and why it is not rejected is still something of a
mystery, despite evidence for a number of possible mechanisms, including
specific suppressor cells, serum blocking and immunosuppressive factors, and
special properties of both placenta (maternal) and trophoblast (fetal).
Xenografts There is considerable interest in the
possibility of using pigs as animal donors for organ transplantation because of
the continuing shortfall of available human organs. However, pig xenografts are
rejected in primates within minutes by a process of hyperacute rejection. This
is due to a combination of preformed antibodies against carbohydrate structures
found in pigs but not primates, and the fact that the complement-regulating
proteins on pig tissue (e.g. DAF; see Fig. 6) do not interact well with human
complement. There is also continuing concern that pigs may harbour novel
retroviruses, which could ‘jump’ the species barrier during transplantation and
cause a new epidemic similar to AIDS.
Organ cloning Because of the continual shortage of donors for
organ transplants, there is enormous excitement about the possibility of
growing ‘designer’ organs by differentiating stem cells (whether embryonic or
non-embryonic) in culture. There has been great progress towards achieving this
remarkable technical feat in animal models. However, as embryonic stem cells
will generally be derived from a different individual than the organ recipient,
the question of immunological rejection remains.
Immunosuppression (for further details
see Fig. 40) Non-specific The success of modern transplantation
surgery is due largely to the introduction of cyclosporin, and later FK506, two
drugs that selectively block the activation of T cells in an antigen non-specific
way (see Fig. 12). These two drugs, together with some cytotoxic drugs, are
used at high concentrations postoperatively to block the initial acute
rejection, and then at lower maintenance doses to block chronic rejection. Some
other approaches are shown in Fig. 40.
Specific suppression is directed at either the antigens
inducing a response or the receptors on the cells carrying it out. When brought
about by antibody, this is conventionally called enhancement and when by
antigen, tolerance. Antigen-specific suppression is the goal of
transplantation immunologists, but has still to be demonstrated in humans.
Regulatory T cells (TREG) can suppress ongoing immune
responses in an antigen-specific way (see Fig. 22). Isolating and expanding
TREG cells in culture and then reintroducing them into a graft recipient has
shown considerable promise in preventing rejection in animal models, and ways
to translate this into clinical treatments are being actively pursued.