Pathogen Recognition
The innate
immune response plays a crucial role in the proin-flammatory response to
infection and relies upon the ability of host defenses to differentiate self
from nonself so that only invading organisms are targeted. The leukocytes
involved in this response recognize certain evolutionarily retained patterns
present on the surface of pathogens and in response bind to the membrane and
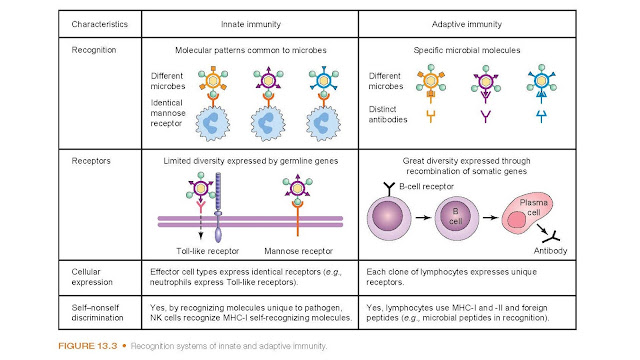
destroy the invading organism through the process of phagocytosis (Fig. 13.3).
Pattern
Recognition
Invading
pathogens contain conserved structures in their cell membranes termed pathogen-associated
molecular patterns (PAMPs), which are recognized by the cells of the innate
immune system because they possess a limited number of germline-encoded
pattern recognition receptors
(PRRs).
Upon PAMP
recognition, PRRs come in contact with the cell surface and/or send
intracellular signals to the host that trigger proinflammatory and
antimicrobial responses including the synthesis and release of cytokines,
chemokines, and cell adhesion molecules. The PAMPs recognized by the host PRRs
are made up of a combination of sugars, lipid molecules, proteins, or patterns
of modified nucleic acids and are essential to the functioning and infectivity
of the pathogen. Because the PAMPs are essential for the functioning of the
microorganism, mutation cannot help it avoid immune recognition. The human
complement of PRRs is very extensive (approximately
1000) so the classes of pathogens recognized by
them are very diverse. Therefore, pathogens of very different biochemical
composition are recognized by relatively similar mechanisms by host PRRs, and
no single class of pathogens is sensed by only one type of PRR. Therefore, the
host genetic code allows for the unique receptors involved in both innate and adaptive
immunity to recognize fine details of molecular structure.
The
ability of the innate immune response to limit microbes early in the infectious
process results from the binding of pathogens to the PRRs on leukocytes, which
in turn initiates the signaling events that lead to complement activation,
phagocytosis, and autophagy. Once initiated, white blood cells, neutrophils,
and monocytes migrate from the blood to the tissues, along with other body
fluids causing peripheral edema. Blood monocytes mature into macrophages as
they traverse the tissues and join the macrophages and DCs already present in
the tissues. PRRs present on these cells become activated, which amplifies the
inflammatory response through enhanced secretion of all chemical mediators including cytokines and complement.
Toll-Like
Receptors
The most
studied PRRs associated with the innate immune response are the Toll-like
receptors (TLRs). TLRs derive their name from the study of the Drosophila
melanogaster toll protein, which is responsible for the resistance of Drosophila
to bacterial and fungal infections. Structurally, TLRs are integral
glycoproteins that possess an extracellular or luminal ligand-binding site
containing leucine-rich repeats and a cytoplasmic signaling toll/interleukin-1
(IL-1) domain. Binding of PAMP to a TLR induces a conformational change in the
receptor, which subsequently triggers intracellular signal transduction and
activation of cellular processes, such as activation of transcription factors
such as nuclear factor κβ (NF- κβ). NF-κβ regulates the production of a number
of proteins that are important components of innate immunity. TLRs can be found
in most of the bone marrow cells including the macrophages, DCs, neutrophils, T
cells, B cells, and non–bone marrow cells including epithelial and fibrocytes.
Eleven different TLRs have been identified in humans, and they each recognize
distinct PAMPs derived from various microorganisms including bacteria, viruses,
fungi, and protozoa.
Human
TLRs can be divided into subfamilies that primarily recognize related PAMPs.
TLR1, TLR2, TLR4, and TLR6 recognize lipids and lipopolysaccharides (LPS),
whereas TLR3, TLR7, TLR8, and TLR9 recognize nucleic acids. TLRs can also be
classified according to their cellular distribution such that TLR1, TLR2, TLR4,
TLR5, TLR6, TLR10, and TLR11 are expressed
extracellularly and THR3, TLR7, TLR8, and TLR9 are mainly expressed in
intracellular compartments. These receptors are involved in responses to widely
divergent types of molecules that are commonly expressed by microbial, but not
mammalian, cell types.
For example, TLR4
is essential for phagocytic
recognition and response to the LPS present in gram-negative
bacteria. TLR2 binds to peptidoglycan, which is
an essential component of the cell wall of gram-positive bacteria. Finally,
TLR5 can recognize the protein flagellin found in flagellated bacteria. In
addition to their role in the immune response, TLRs have been shown to have a pathologic
role in disorders such as atherosclerosis, allergies, and certain autoimmune diseases.