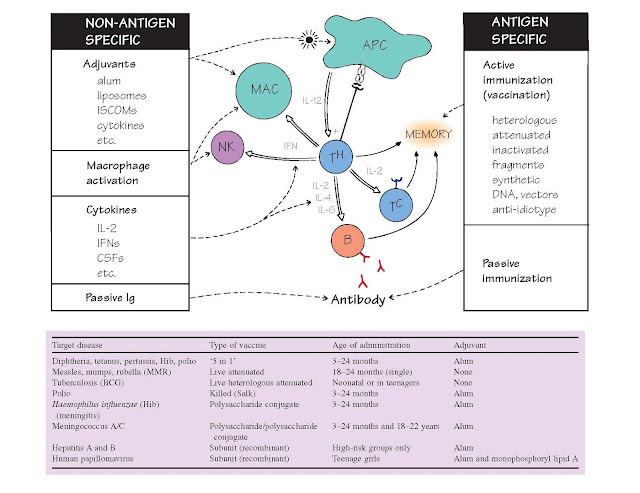
Immunostimulation And Vaccination
In most animals the combination of innate
resistance and stimulation of adaptive
responses by antigen is adequate to cope with common infections (otherwise the
species would not survive!). However, the immune system does have its
shortcomings, and some of these can be overcome by artificial means. Indeed,
the introduction of vaccines has probably saved more lives than any other
medical intervention to date. But there are still no effective vaccines against
many of the world’s most common infectious diseases, including HIV,
tuberculosis and malaria.
Most effective vaccines need to
stimulate both innate and adaptive immunity. Adaptive immune responses suffer
from their initial slow- ness, so that high levels of antibody may arrive too
late to prevent death or disability (e.g. tetanus, polio) even though surviving
patients are resistant to reinfection. Specific immunization overcomes
this problem by ensuring there is a high level of immunity before exposure.
This may be active (top right), in which antigen is used to safely
generate immunological memory, aided in some cases by the boosting power of
special non-specific stimulants or
adjuvants (top left), or passive, in which preformed antibody is injected, with more rapid
but short-lived effect. Immunotherapy, as distinct from vaccination, refers to
stimulating immune responses to cure, rather than prevent, disease. In general,
conventional vaccines are ineffective when administered after exposure,
although there are exceptions (rabies, chickenpox vaccination for prevention of shingles). Finally, when some component of the immune
system is deficient (see Fig. 33), efforts can be made to correct this by replacement
of hormones, enzymes, cytokines, cells or organs.
Despite 200 years of cumulative
success, there is a growing irrational fear of vaccination in the
industrialized world and a corresponding rise in cases of dangerous illnesses
such as measles and polio. Continued efforts at educating the public are
required to ensure society benefits
fully from the benefits of universal vaccination.
Adjuvants are materials that increase the response to an antigen given at the same time. One way in which many
adjuvants work is by creating a slow-release depot of antigen, thus prolonging
the time for which the immune system remains in contact with antigen. In
addition, they contain substances that activate macrophages and dendritic cells
and via this pathway also increase antigen presentation (see Fig. 18). The most
powerful adjuvants (e.g. Freund’s complete, which contains extracts of Mycobacterium
tuberculosis) are too tissue-destructive for human use. Most human vaccines
use a mixture of insoluble aluminium salts (alum) as adjuvant, but considerable
efforts are being made to find more effective alternatives such as saponin.
Replacement therapy In some cases of severe combined immunodeficiency,
bone marrow grafting has restored function; where adenosine deaminase (ADA) is
deficient, this enzyme may also be restored by blood transfusion or, more
recently, by gene therapy.
Cytokines Interferons, interleukins and other cytokines
have potential for increasing the activity of their target cells, but their use
in the clinic has been limited. IFNα has proved useful in certain viral dis-
eases (e.g. hepatitis B and C), while G-CSF is used to boost granulocyte
numbers after radiation or chemotherapy. However, the side effects of administering
large amounts of cytokines systemically often limit their usefulness. More
targeted cytokine release, e.g. by gene therapy, may prove more effective.
Antibody In patients already exposed to disease,
passively transferred antibody antiserum may be life-saving; examples are
rabies, tetanus, hepatitis B and snake bite. Originally, antisera were raised
in horses, but the danger of serum sickness (see Fig. 36) makes ‘humanized’
monoclonal antibodies (see Fig. 15) preferable wherever possible. Monoclonal
antibodies against ‘self’ molecules have also proved remarkably effective in
controlling some tumours (see Fig. 42). T cells are more difficult to
administer, because they need to be obtained from the same individual to
prevent rejection. However, T cells against cytomegalovirus, which are isolated
from blood, stimulated with virus and cytokines, and then readminstered to the
patient, have proved useful in controlling this infection in immunosuppressed
individuals (e.g. after
transplantation).
The term ‘vaccine’ was introduced
by Pasteur to commemorate Jenner’s classic work with cowpox (vaccinia), but was
extended by him to all agents used to induce specific immunity and mitigate the
effects of subsequent infection. Vaccines are given as early as practical,
taking into account the fact that the immune system is not fully developed in
the first months of life, and that antibody passively acquired from the mother
via the placenta and/or milk will specifically prevent the baby making its own
response. In general, this means a first injection at about 6 months, but where
antibody is not of major importance (e.g. BCG) vaccines can be given effectively
within 2 weeks of birth.
Living heterologous vaccines work by producing a milder but
cross-protecting disease; one example is vaccinia, which has effectively
allowed the elimination of smallpox. Another is BCG (attenuated bovine
tuberculosis), which provides partial protection against tuberculosis
especially when given to infants. However, with the rapid rise in tuberculosis
worldwide, improved vaccines are urgently needed.
Living attenuated viruses (measles, mumps, yellow fever, rubella)
produce subclinical disease and usually excellent protection. However, care is
needed in immunodeficient patients. The measles, mumps and rubella vaccines are
usually administered together (MMR). Public confidence in this vaccine was
severely damaged by flawed research claiming a link between the vaccine and
autism.
Toxoids are bacterial toxins (e.g. diphtheria, tetanus)
inactivated with formalin but still antigenic. These relatively simple vaccines
have provided some of the most effective and reliable vaccines available to
this day.
Capsular polysaccharides induce some (primarily IgM) antibody against
meningococcal, pneumococcal and Haemophilus spp. infection. However, the
level and persistence of protective antibody can be greatly enhanced by
coupling the polysaccharide to protein antigens, which stimulate a strong
‘helper’ response. Tetanus or diphtheria toxoid is frequently used for this
purpose. These ‘conjugate’ vaccines have proved of particular value in the
fight against bacterial meningitis.
Subunit vaccines include the first of the ‘second-generation’
vaccines, in which the purified antigens are produced by recombinant DNA
technology. The first examples of subunit vaccines were hepatitis A and B
surface antigens and they provide a high (>90%) level of protection. A
recombinant surface antigen vaccine against the sexually transmitted human
papillomavirus was introduced in 2007 and pre- vents both viral infection and
the subsequent development of cancer of the cervix, which is caused by this
virus.
DNA, vectors An interesting idea is to insert genes from one
microbe into another less virulent one such as vaccinia, attenuated Salmonella
or even HIV-based ‘viruses’ which have been altered so as to prevent them
replicating. These ‘recombinant’ organisms often stimulate strong immunity to
the inserted antigens. If the vector has a large enough genome (e.g. BCG),
multiple antigens could be introduced into a single vector, cutting down the
need for repeated doses. A recent trial of such a ‘recombinant’ vaccine gave
the first suggestion of protection against HIV infection. Some of the
properties of the vaccines in common use are summarized in the table opposite
(representing 2012 UK guidelines).