Cancer Immunology.
It has
long been suspected that the immune system may be able to recognize tumours and destroy them, as it does
an allogeneic transplant or a parasite. There is now good evidence for the old
hypothesis that naturally occurring tumours are eliminated or contained by the
immune system (‘immune surveillance’). This hypothesis predicts that the
frequency or progression of tumours increases in immunosuppressed individuals,
a prediction that was initially borne out by studies on virally induced
tumours, but has recently been extended to other more common types.
Immunologists have therefore hoped that by appropriate stimulation of stronger
innate or specific immunity (vaccination) the immune system could contribute to
the eradication of cancer. In the last few years the enormous effort devoted to
this problem has begun to be translated into some clinical successes and the
mood is one of cautious optimism.
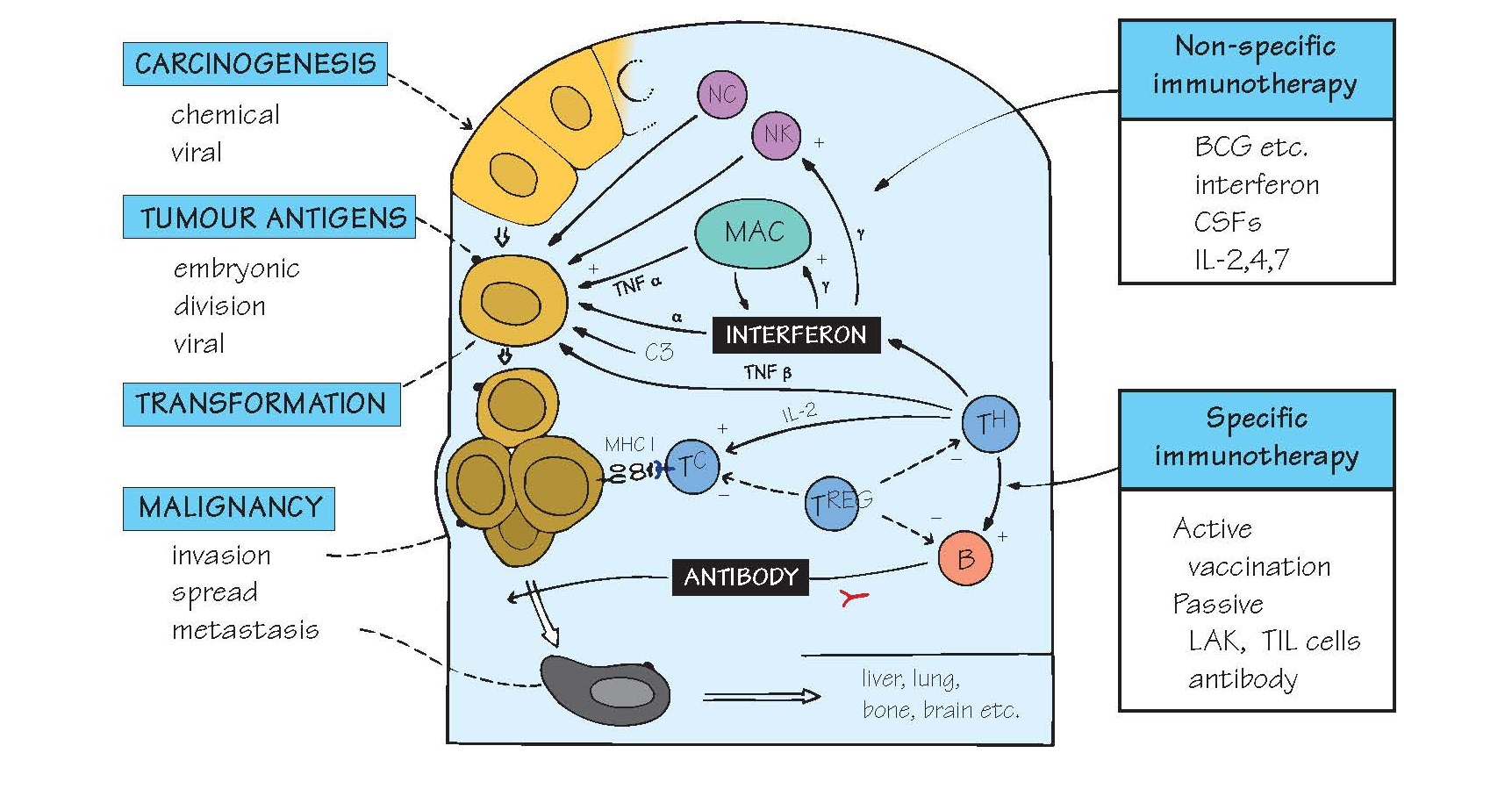
Many mechanisms can contribute to
tumour control, including those of both innate (e.g. NK cells, macrophages,
cytokines) and adaptive immunity. Attention has been focused on the
identification of tumourspecific B- and T-cell antigens, although it now seems
likely that tumouassociated antigens (TAAs), normal proteins found more frequently
or at higher levels on tumour cells than on normal tissues, are equally
important. Older research concentrated on the study of experimentally induced
tumours in animals, but probably these very fast-growing and aggressive
tumours are much easier for the immune system to recognize than the more
typical human tumours that usually develop gradually over years or even
decades. Instead, attempts are being
made to identify the naturally occurring immune responses to tumours in patients with cancer.
Nevertheless, tumours continue to
pose formidable challenges to the immunologist. In its relationship to the
host, a tumour cell (yellow, brown and black in figure) is rather like a
successful parasite, but with special additional features. Parasite-like
mechanisms that help prevent elimination include weak antigenicity and
extensive cross-reaction with self; immunosuppression and tolerance
induction; release of soluble antigens; antigen antibody complexes;
and antigenic variation.
In mice, chemicals such as
methylcholanthrene and benzopyrenes tend to induce tumours, each with unique
‘idiotypic’ antigens, whereas most of the common human cancers result from a
slow and gradual accumulation of mutations in the genes of proteins that
regulate the cell cycle. Such mutations can result in over-activation of a
protein promoting cell growth (encoded by cellular oncogenes) or
inactivation of a protein that normally slows down cell growth (encoded by tumoursuppressor
genes). Some of these mutations are inherited, while others may result from
exposure to chemicals in the environment. Normally, it requires several
mutagenic events, which can occur over many decades, before a tumour develops.
The mutated forms of these proteins may act as possible specific antigens for
the adaptive immune system,
especially the cytotoxic T cell.
BCG (an attenuated tubercle bacillus) has been
tried against melanoma, sarcoma, etc., especially combined with other
treatments. Its major immunological effect seems to be macrophage activation,
but it may also affect NK cells. A tremendous range of bacterial and other
immunostimulating agents has been tested for antitumour activ- ity (see Fig.
41), but so far with very limited success.
MAC, NK Macrophages and natural killer cells (see Figs
8 and 15), especially when activated,
can prevent growth
of some tumours in vitro (‘cytostasis’) or
actually kill them (‘cytolysis’). NK cells are also cytotoxic, and are
activated by cells that have lost expression of MHC molecules, a common
phenotype of many tumours. IFNγ is important in activating macrophages and NK
cells. Some tumour cells can apparently activate complement via the
alternative pathway. However, note that there are potential dangers of
activating macro- phages and inflammation as discussed in the paragraph above.
Lymphocytes Tumours often contain large numbers of tumour-
infiltrating lymphocytes (TILs), and the number and type of these cells can
sometimes predict the rate of tumour progression. TILs are believed to be
enriched for lymphocytes specifically recognizing the tumour cells, and such
cells extracted from the tumour itself, expanded and then reinjected, have in
some cases been successful in causing tumour rejection. Lymphocytes from the
blood of tumour patients, activated non-specifically in vitro by IL-2 to
kill (LAK cells) have also shown some promise.
Tumour antigens In the case of tumours induced by viruses, the viral
antigens themselves are the target of the host immune response (see below).
In non-viral tumours, the identification of TAAs has been much more difficult.
In rare cases, embryonic antigens absent from normal adult cells may be
re-expressed when they become malignant. Carcinoembryonic antigen (CEA) in the
colon and α-fetoprotein in the liver are examples of diagnostic value. Other
antigens found on the surface of some tumours are glycosylation variants of
normal cell proteins (e.g. MUC-1 on epithelial tumours). However, it seems that
the majority of antigens recognized by the host’s cellular immune response are
normal self proteins, which are expressed at higher concentrations than normal
in the tumour cells (sometimes because they are required for cell division).
Unfortunately, it seems as though tumours are very heterogeneous and antigens
common to a large number of tumours have been difficult to identify.
Viruses were once thought to be responsible for many
human tumors, but most common cancers are now thought not to be virally
induced. However, five important forms of cancer are firmly linked to viruses
(all DNA): Burkitt’s
lymphoma and nasopharyngeal carcinoma (EBV), Kaposi’s sarcoma
(KSHV), hepatocarcinoma (hepatitis
B virus [HBV]) and cervical
cancer (papillomavirus). RNA retroviruses may be responsible for some other
cases. Interestingly, all these tumours increase in frequency in
immunosuppressed individuals (Kaposi’s sarcoma, for example, is commonly found
in AIDS patients; see Fig. 28). Marek’s disease, a tumour of chickens, was the
first example of the introduction of a successful tumour vaccine. HBV
vaccination lowers the risk of hepatocellular carcinoma by preventing viral
infection, and a vaccine against papillomavirus prevents most cases of cervical
cancer
Antibody There is little evidence that antibody normally
provides any host immunity to tumours. Nevertheless, passive immunization using
antibodies against two TAAs, CD20 on B-cell lymphomas and Her2/ neu on
epithelial cells, has been the first major success of tumour immunology, and
these have entered the standard repertoire of drugs used by oncologists for
treating these diseases. Much effort is under- way to extend these successes to
other tumours, and several other antibodies are in advanced stages of clinical
trial. Another approach is to enhance the effectiveness of antibodies by
coupling them to potent cytotoxic drugs (‘magic bullet’). This aims to build up
very high levels of anti-cancer drug in the immediate vicinity of the tumour,
thus minimizing the general toxicity of the drug, which limits the
concentrations that can normally be used for chemotherapy.
Cell-mediated immunity Cytotoxic CD8 T cells capable of lysing tumour
cells in vitro have been isolated from both mice and humans (especially
from individuals with melanoma). In mice such cytotoxic T cells can eliminate a
tumour in vivo. Many tumours evade this by reducing their expression of
MHC class I antigens. TH1 cells are also probably very important, because they
can activate macrophages and NK cells via the release of IFNγ and are also
essential for good CD8 T-cell memory. However, weak T-cell reactions may
actually stimulate tumour growth and metastasis. Recent promising clinical vaccination
trials using melanoma antigens have given a strong further impetus to this
work, and there is also the possibility that T cells could be ‘redirected’
against a target tumor antigen by gene therapy of specific T-cell receptors
(see Fig. 12).
Dendritic cells (see Fig. 4) are the most potent activators of
cell- mediated immunity and it is therefore not surprising that many approaches
have attempted to harness these cells for immunotherapy. One approach is to
isolate dendritic cells from a patient, load them with tumour antigens and then
reintroduce them into the body. Although these patient-specific adoptive
immunotherapy procedures are difficult and expensive to implement, a
dendritic cell-based immunotherapy for prostate cancer has recently been
licenced in the USA, and further treatments of this type are likely to follow.
Breaking tolerance The immune response to most tumours is probably
limited by the strong regulatory mechanisms that operate to prevent
autoimmunity and maintain tolerance (see Fig. 22). Many strategies aimed at
interfering with these mechanisms, and hence obtaining more effective immune
responses are being explored. These include blocking molecules on the T-cell
surface such as CTLA4 which transmit negative signals, depleting TREG cells and
using gene therapy to produce large populations of T cells that carry specific
receptors for tumour antigens. New biological drugs based on these strategies
are now entering the clinic, and there is great excitement about their
potential. Note that treatments may involve some unavoidable side effects in
the form of autoimmunity (see Fig. 38).