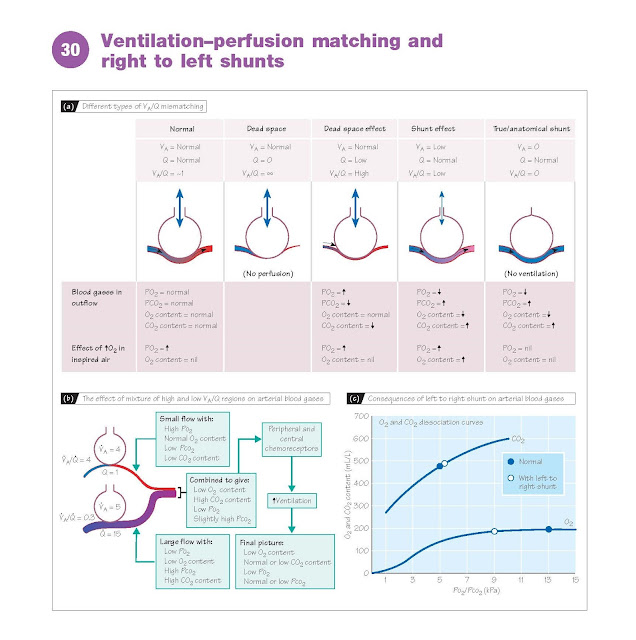
At rest, total alveolar ventilation
(VA) is similar to total pulmonary capillary perfusion (Q), or about 5 L/min. For
optimal gas exchange, all regions of the lung should ideally have a ventilation–perfusion
ratio (VA/Q) of unity. When there are variations significantly away from unity,
either lower or higher, this is referred to as ventilation– perfusion mismatch.
In a right to left shunt (see below), for example, ventilation is zero and
VA/Q = ∞; whereas when an embolism blocks a pulmonary artery, perfusion in the part
of the lung fed by that artery is zero, and VA/Q = 0. Regions of the lung that have
a VA/Q value much greater than unity have excessive ventilation, and blood derived
from them will have a high Po2 and a low Pco2 (dead space effect). Regions
with a VA/Q value much less than unity have a shunt or venous admixture
effect; there is some gas exchange, but the blood has a lower than normal Po2
and a higher than normal Pco2.
Effect of ventilation–perfusion mismatch
on arterial gases. Regions
of high VA/Q cannot compensate for regions
of low VA/Q. This is because of the way in which O2 is carried in the blood (Chapter
28). Although regions of high VA/Q produce blood with a high Po2, this does not
translate to any significant increase in O2 content, as the haemoglobin in the blood
is already close to saturation at the normal Po2. Conversely, blood derived from
regions with a low VA/Q, especially if Po2 < 8 kPa, will have a significantly
reduced O2 content (Fig. 30c). Regions of high VA/Q are also most usually due to
insufficient perfusion, so that the amount of blood, and therefore O2, that such
regions contribute to the total will be relatively small. As a result, the
combined blood from regions with high and low VA/Q will have a low O2 content and
a low Po2, even if total ventilation and perfusion are matched for the whole lung.
The CO2 content is less severely affected,
because overventilated areas can lose extra CO2 and partly compensate for underventilated
areas. Moreover, a rise in Pco2 will stimulate breathing via the chem-
oreceptors, allowing CO2 to be corrected, or even overcorrected if Po2 is sufficiently
low (Chapter 29). Significant VA/Q mismatching will therefore usually result in
arterial blood with a low Po2 but normal or low Pco2 (Fig. 30b). Ventilation with
O2-enriched air will improve oxygenation in regions of low VA/Q, but is not useful
for shunts, as the enriched air never reaches the shunted blood. Hypoxic pulmonary
vasoconstriction (Chapter 24) reduces the severity of VA/Q mismatch by diverting
blood from the affected region to well-ventilated areas.
Effect of gravity
The blood pressure at the base of the
lungs is greater than that at the apex (top) because of the weight of the column
of blood. This increased pressure distends
the pulmonary blood vessels at the base and the flow is therefore increased. Conversely, blood flow at
the apex may be reduced if the pulmonary venous pressure falls below the alveolar
pressure, when the vessels will be compressed. The net result is that, on standing,
pulmonary blood flow falls progressively on moving from the bottom of the lung to
the top.
Gravity also affects the intrapleural
pressure, which is thus less negative at the base of the lung than at the apex.
Alveoli at the base are therefore less expanded at functional residual capacity,
and thus have more potential for expansion during inspiration. As a result,
ventilation is greatest at the base of the lung. Although the effects of
gravity on perfusion and ventilation partly cancel each other out, ventilation is
less affected than perfusion, so that VA/Q is highest at the apex of the lung and
lowest at the base. In the young, this relatively small variation has little effect
on blood gases, but in the elderly, it may contribute to a low Po2.
Right to left shunts
Part of the venous effluent of the bronchial
and coronary circulations bypasses the lungs and enters the pulmonary vein and left
ventricle, respectively (Chapter 16). Oxygenated blood from the lungs is there-
fore diluted by venous blood. These are anatomical right to left shunts and
account for <2% of cardiac output in healthy individuals. Larger shunts can occur
in disease when regions of the lung are not ventilated (e.g. lung collapse, pneumonia),
or due to congenital heart malformations. When calculating the effects of
right to left shunts on arterial blood, the blood content of O2 and CO2 needs
to be considered. For a 20% shunt, the 80% of blood passing through the lungs will
have normal arterial O2 and CO2 contents of 200 and 480 mL/L, respectively, whilst
the 20% bypassing the lungs will have normal venous values of 150 and 520 mL/L,
respectively. On combination, the blood will contain (200 × 0.8) + (150 × 0.2) =
190 mL/L of O2, and (480 × 0.8) + (520 × 0.2) = 488 mL/L of CO2. From the dissociation
curves (Fig. 30c), it can be seen that this results in a fall in Po2 from 13 to
9 kPa, whereas Pco2 rises only marginally from 5.3 to 5.5 kPa because of the steeper
curve. Changes in Pco2 and Po2 stimulate the chemoreceptors and increase ventilation
(Chapter 29), so that arterial Pco2 returns to normal. However, increased ventilation
cannot increase blood O2 content, as the haemoglobin of the blood passing through
the lungs is already close to saturation. Thus, right to left shunts commonly result
in a low arterial PO2 but a normal
or low PCO2.