Muscle Tissue.
Muscle tissue, whose primary
function is contraction, is responsible for movement of the body and its parts
and for changes in the size and shape of internal organs. Muscle tissue
contains two types of fibers that are responsible for contraction: thin and
thick filaments. The thin filaments are composed primarily of actin, whereas
the thick filaments are composed of myosin. The two types of myofilaments
occupy the bulk of the cytoplasm, which in muscle cells is called the sarcoplasm.
There are three types of muscle
tissues: skeletal, cardiac, and smooth. Skeletal and cardiac
muscles are striated muscles, in which the actin and myosin filaments are
arranged in large, parallel arrays in bundles, giving the muscle fibers a
striped or striated appearance when observed with a microscope. Smooth
muscle lacks striations and is found in the iris of the eye, the walls of
blood vessels, hollow organs such as the stomach and urinary bladder, and
hollow tubes, such as the ureters and common bile duct, that connect internal
organs.
Neither skeletal nor cardiac muscle
can undergo the mitotic activity needed to replace injured cells. Smooth muscle,
however, may proliferate and undergo mitotic activity. Some increases in smooth
muscle are physiologic, as occurs in the uterus during pregnancy. Other
increases, such as the increase in smooth muscle that occurs in the arteries of
persons with chronic hypertension, are pathologic.
Although the three types of muscle
tissue differ significantly in structure, contractile properties, and control mechanisms,
they have many similarities. In the following section, the structural
properties of skeletal muscle are presented as the prototype of striated muscle tissue. Smooth muscle and the ways in which it differs from skeletal
muscle are also discussed.
Skeletal Muscle
Skeletal muscle is the most abundant tissue in the body,
accounting for 40% to 45% of the total body weight. Most skeletal muscles are attached to bones,
and their contractions are responsible for movements of the skeleton. Each
skeletal muscle is a discrete organ made up of hundreds or thousands of muscle
fibers. At the periphery of skeletal muscle fibers, randomly scattered
satellite cells are found. They represent a source of undifferentiated myoblast
cells that may be involved in the limited regeneration capabilities of skeletal
muscle. Although muscle fibers predominate,
substantial amounts of connective
tissue, blood vessels, and nerve fibers are also present.
Organization and Structure. In an intact muscle, several different
layers of connective tissue hold the individual muscle fibers together.
Skeletal muscles such as the biceps brachii are surrounded by a dense,
irregular connective tissue covering called the epimysium (Fig. 4.22A).
Each muscle is subdivided into smaller bundles called fascicles, which
are surrounded by a connective tissue covering called the perimysium.
The number of fascicles and their size vary among muscles. Fascicles consist of
many elongated structures called muscle fibers, each of which is
surrounded by connective tissue called the endomysium. Skeletal muscles
are syncytial or multinucleated structures, meaning there are no true cell
boundaries within a skeletal muscle fiber.
The sarcoplasm of the muscle fiber
is contained within the sarcolemma, which represents the cell membrane.
Embedded throughout the sarcoplasm are the contractile elements actin and
myosin, which are arranged in parallel bundles called myofibrils. The
thin, lighter-staining myofilaments are composed of actin, and the thicker,
darker-staining myofilaments are composed of myosin. Each myofibril consists of
regularly repeating units along the length of the myofibril, called sarcomeres
(see Fig. 4.22B).
Sarcomeres are the structural and
functional units of cardiac and skeletal muscle. A sarcomere extends from one Z
line to another Z line. Within the sarcomere are alternating light and dark
bands. The central portion of the sarcomere contains the dark band (A band)
consisting mainly of myosin filaments, with some overlap with actin filaments.
Straddling the Z line, the lighter I band contains only actin filaments; there-
fore, it takes two sarcomeres to complete an I band. An H zone is found in the
middle of the A band and represents the region where only myosin filaments are
found. In the center of the H zone is a thin, dark band, the M band or M line,
produced by linkages between the myosin filaments. Z lines consist of short
elements that interconnect and provide the thin actin filaments from two
adjoining sarcomeres with an anchoring point.
The sarcoplasmic reticulum, which
is comparable to the
smooth ER, is
composed of longitudinal
tubules that run parallel to the muscle fiber and surround
each myofibril (see Fig. 4.22D).
This network ends in enlarged, saclike regions called the lateral sacs or
terminal cisternae. These sacs store calcium that is released during
muscle contraction. A binding protein called calsequestrin found in the
terminal cisternae enables a high concentration of calcium ions to be
sequestered in the cisternae.9 Concentration levels of calcium ions in the
cisternae are 10,000 times higher than in the sarcoplasm.
A second system of tubules consists
of the transverse or T tubules, which are extensions of the
plasma membrane and run perpendicular to the muscle fiber. The hollow portion
or lumen of the transverse tubule is continuous with the extra-cellular fluid
compartment. Action potentials, which are rap- idly conducted over the surface
of the muscle fiber, are in turn propagated by the T tubules into the
sarcoplasmic reticulum. As the action potential moves through the lateral sacs,
the sacs release calcium, initiating muscle contraction. The membrane of the
sarcoplasmic reticulum also has an active transport mechanism for pumping
calcium back into the reticulum. This prevents interactions between calcium
ions and the actin and myosin myofilaments after cessation of a muscle
contraction.
Skeletal Muscle Contraction. During muscle contraction, the thick myosin and
thin actin filaments slide over each other, causing shortening of the muscle
fiber, although the length of the individual thick and thin filaments remains
unchanged (see Fig. 4.22C). The structures that produce the sliding of the
filaments are the myosin heads that form cross-bridges with the thin actin
filaments (Fig. 4.23). When activated by ATP, the cross-bridges swivel in a
fixed arc, much like the oars of a boat, as they become attached to the actin
filament. During contraction, each
cross-bridge undergoes its own cycle of movement, forming a bridge attachment and releasing it, and moving to another site where the same sequence
of movement occurs. This pulls the thin and thick filaments past each other.
Myosin is the chief constituent of
the thick filament. It consists of a thin tail, which provides the structural
backbone for the filament, and a globular head. Each globular head contains a
binding site able to bind to a complementary site on the actin molecule.
Besides the binding site for actin, each myosin head has a separate active site
that catalyzes the breakdown of ATP to provide the energy needed to activate
the myosin head so that it can form a cross-bridge with actin. After
contraction, myosin also binds ATP, thus breaking the linkage between actin and
myosin. Myosin molecules are bundled together side by side in the thick
filaments such that one half have their heads toward one end of the filament
and their tails toward the other end; the other half are arranged in the
opposite manner.
The thin filaments are composed
mainly of actin, a globular protein lined up in two rows that coil around each
other to form a long helical strand. Associated with each actin filament are
two regulatory proteins, tropomyosin and troponin (see Fig. 4.23A). Tropomyosin,
which lies in grooves of the actin strand, provides the site for attachment
of the globular heads of the myosin filament. In the noncontracted state, troponin
covers the tropomyosin-binding sites and prevents formation of
cross-bridges between the actin and myosin. During an action potential, calcium
ions released from the sarcoplasmic reticulum diffuse to the adjacent
myofibrils, where they bind to troponin. Binding of calcium to troponin
uncovers the tropomyosin-binding sites such that the myosin heads can attach
and form cross-bridges. Energy from ATP is used to break the actin and myosin
cross-bridges, stopping the muscle contraction. After the linkage between actin
and myosin is broken, the
concentration of calcium around the myofibrils
decreases as calcium is actively transported into the sarcoplasmic reticulum by
a membrane pump that uses energy derived from ATP.
The basis of rigor mortis can
be explained by the binding of actin and myosin. As the muscle begins to
degenerate after death, the sarcoplasmic cisternae release their calcium ions,
which enable the myosin heads to combine with their sites on the actin
molecule. As ATP supplies diminish, no energy source is available to start the
normal interaction between actin and myosin, and the muscle is in a state of
rigor until further degeneration destroys the cross-bridges between actin and
myosin.6
Smooth Muscle
Smooth muscle is often called involuntary
muscle because its activity arises spontaneously or through activity of the
autonomic nervous system. Smooth muscle contractions are slower and more
sustained than skeletal or cardiac muscle contractions.
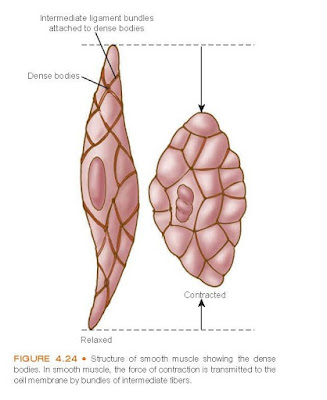
Organization and Structure. Smooth muscle cells are spindle shaped and
smaller than skeletal muscle fibers. Each smooth muscle cell has
one centrally positioned
nucleus. Z lines and M lines are not present in smooth muscle fibers,
and cross-striations are absent because the bundles of filaments are not
parallel but crisscross obliquely through the cell. Instead, the actin filaments
are attached to structures called dense bodies (Fig. 4.24). Some dense
bodies are attached to the cell membrane, and others are dispersed in the cell
and linked together by structural
proteins.
The
lack of Z lines and the regular overlapping of contractile elements provide a
greater range of tension development. This is important in hollow organs that
undergo changes in volume, with consequent changes in the length of the smooth
muscle fibers in their walls. Even with the distention of a hollow organ, the
smooth muscle fiber retains some ability to develop tension, whereas such
distention would stretch skeletal muscle beyond the area where the thick and thin
filaments overlap.
Smooth muscle is usually arranged
in sheets or bundles. In hollow organs, such as the intestines, the bundles are
organized into the two-layered muscularis externa consisting of an outer,
longitudinal layer and an inner, circular layer. A thinner muscularis mucosae
often lies between the muscularis externa and the endothelium. In blood
vessels, the bundles are arranged
circularly or helically around the vessel wall.
Smooth Muscle Contraction. As with cardiac and skeletal muscle, smooth muscle contraction is initiated
by an increase in intracellular calcium. However, smooth muscle differs from
skeletal muscle in the way its cross-bridges are formed. The sarcoplasmic
reticulum of smooth muscle is less developed than in skeletal muscle, and no
transverse tubules are present. Smooth muscle relies on the entrance of
extracellular calcium and its release from the sarcoplasmic reticulum for
muscle contraction. This dependence on movement of extracellular calcium across
the cell membrane during muscle contraction is the basis for the action of
calcium-blocking drugs used in the treatment of cardiovascular disease.
Smooth muscle also lacks troponin,
the calcium-binding regulatory protein found in skeletal and cardiac muscle.
Instead, it relies on another calcium-binding protein called calmodulin.
The calcium–calmodulin complex binds to and activates the myosin-containing
thick filaments, which interact with actin.
Types of Smooth Muscle. Smooth muscle may be divided into two broad
categories according to the mode of activation: multiunit and single-unit
smooth muscle. In multiunit smooth muscle, each unit operates almost
independently of the others and is often enervated by a single nerve, such as
occurs in skeletal muscle. It has little or no inherent activity and depends on
the autonomic nervous system for its activation. This type of smooth muscle is
found in the iris, in the walls of the vas deferens, and attached to hairs in
the skin. The fibers in single-unit smooth muscle are in close contact
with each other and can contract spontaneously without nerve or hormonal
stimulation. Normally, most of the muscle fibers contract synchronously, hence
the term single-unit smooth muscle. Some single-unit smooth muscle, such
as that found in the gastrointestinal tract, is self-excitable. This is usually
associated with a basic slow-wave rhythm transmitted from cell to cell by nexuses
(i.e., gap junctions) formed by the fusion of adjacent cell membranes.
The cause of this slow-wave activity is unknown. The intensity of contraction
increases with the frequency of the
action potential. Certain hormones, other agents, and local factors can modify smooth muscle activity by
depolarizing or hyperpolarizing
the membrane. Smooth muscle cells found in the uterus and small-diameter blood
vessels are also singleunit smooth muscle.