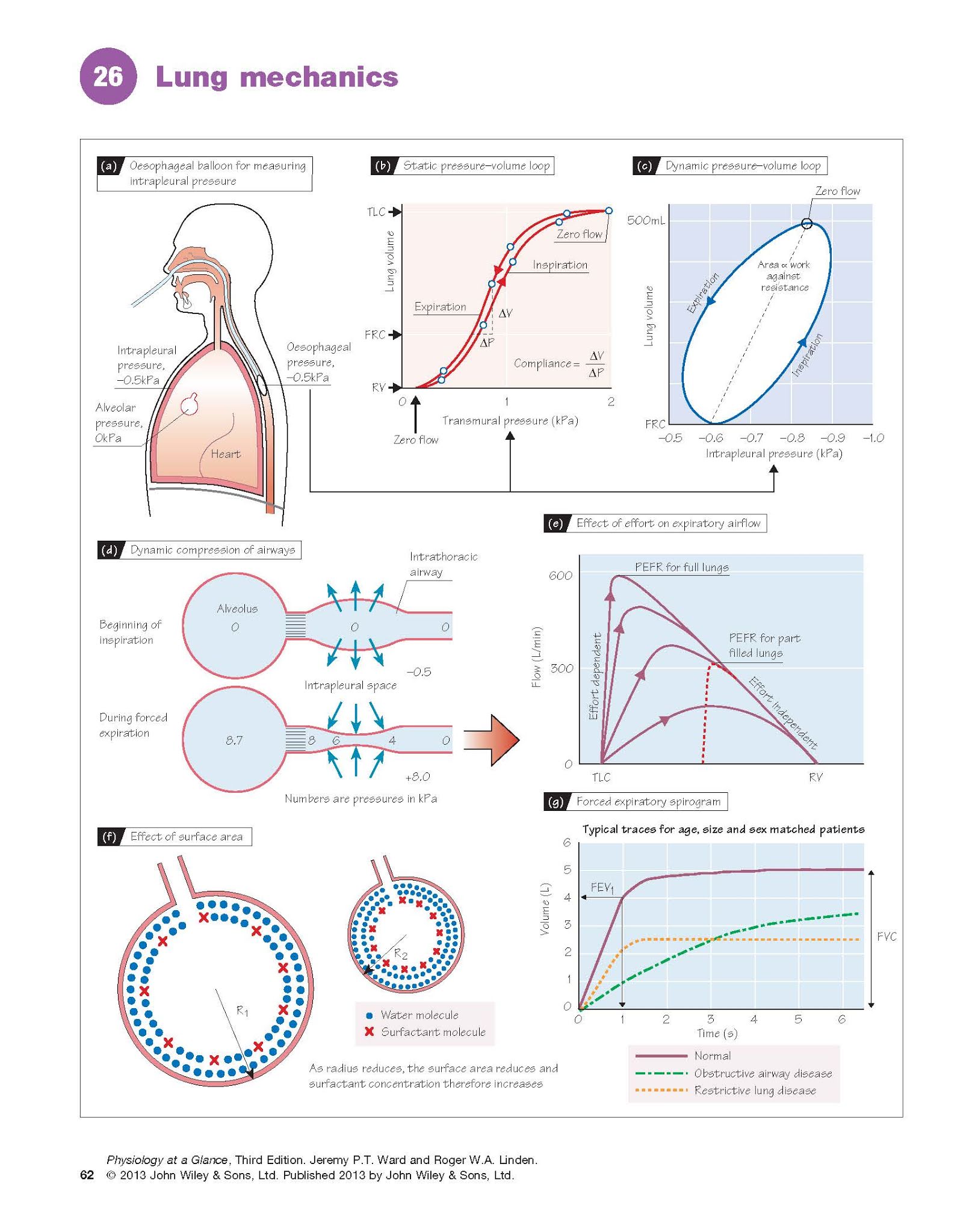
The
respiratory muscles have to overcome resisting forces during breathing. These
are primarily the elastic resistance in the chest wall and lungs, and
the resistance to air flow (airway resistance).
Lung Compliance
The static compliance (‘stretchiness’)
of the lungs (CL) is defined as the change in volume per unit change
in distending pressure (CL = ΔV/ΔP) when there is no air
flow. The distending pressure is the transmural (alveolar–intrapleural)
pressure (Chapter 25). The intrapleural pressure can be measured with an
oesophageal balloon (Fig. 26a). The alveolar pressure is the same as the mouth
pressure (i.e. zero) if no air is
flowing. The subject breathes in steps and the intra-pleural pressure is
measured at each held volume. A typical static pressure–volume plot is
shown in Fig. 26b. The inspiratory and expiratory curves are slightly different
(hysteresis), typical for elastic systems. The static lung compliance
is the maximum slope, generally just above the functional residual capacity
(FRC), and is normally ∼1.5 L/kPa, although this is dependent on age,
size and sex. The static compliance
is reduced by lung fibrosis (stiffer lungs).
The dynamic compliance is
measured during continuous breathing, and therefore includes a component due to
airway resistance. The dynamic pressure–volume loop (Fig. 26c) has a
point at each end where the flow is zero; the slope of the line between these
points is the dynamic compliance. This is normally similar to the static
compliance, but can be altered in disease. The width of the curve reflects the
pressure required to suck in or expel air; the area of the curve is
therefore a measure of the work done against airway resistance.
Surfactant and the alveolar
air–fluid interface
The surface tension of the
fluid lining the alveoli contributes to lung stiffness, as the attraction of
water molecules at the air–fluid interface tends to collapse the alveoli. This
is a manifestation of Laplace’s law (Chapter 11), which shows that the
pressure in a bubble (or alveolus) is proportional to the surface tension (T)
and radius (P ∝ T/r). A small bubble will therefore have a higher pressure than a
larger one and, if connected, will collapse into it. The inward force created
by this surface tension also tends to suck fluid into the alveoli (transudation).
In the lung, these problems are minimized by surfactant, secreted by type
II pneumocytes (Chapter 25). Surfactant is a mixture of phospholipids that
floats on the alveolar fluid surface, and reduces surface tension. As the
alveoli shrink, the effective concentration of surfactant increases, further
lowering the surface tension (Fig. 26f). This more than balances the effect of
reducing radius (as r falls, so does T). Surfactant also reduces lung stiffness
and transudation. Premature babies may not have sufficient surfactant and develop
neonatal respiratory distress syndrome, with stiff lungs, lung collapse
and transudation.
Airway resistance
Flow through the airways is
described by Darcy’s law; flow = (P1 − P2)/R (Chapter 11), where P1 is the alveolar pressure, P2 is the mouth pressure and R is the resistance
to air flow. The airway resistance is determined by the airway radius, according to Poiseuille’s law,
and whether the flow is laminar or turbulent (Chapter 11).
The airway resistance is increased
by factors that constrict the airway smooth muscle (bronchoconstrictors).
These include the reflex release of muscarinic neurotransmitters from
parasympathetic nerve endings, generally due to the activation of irritant
receptors (Chapter 29), and numerous mediators released by inflammatory cells (e.g. histamine, prostaglandins,
leukotrienes), e.g. in asthma. Increased mucus production also
narrows the lumen and increases the resistance. Sympathetic stimulation,
adrenaline (epinephrine) and salbutamol cause relaxation and bronchodilatation
via β2-adrenoceptors on the
smooth muscle.
Effect of transmural pressure. Expiration is normally passive (Chapter 29).
Forced expiration increases the intrapleural and thus alveolar pressure,
increasing the pressure gradient to the mouth and therefore theoretically
leading to increased flow. However, although expiration from fully inflated
lungs is indeed effort dependent, towards the end of the breath,
increasing force does not increase flow, i.e. it is effort independent (Fig.
26e). This occurs as a
result of the pressure gradient between the alveoli and the mouth. Midway
between them, generally in the bronchi, the pressure in the airway falls
below the intrapleural
pressure, causing the
airway to collapse (dynamic compression; Fig. 26d). As there is now
no flow, the pressure rises again until it is greater than the intrapleural
pressure, and the airway re-opens. This sequence happens repeatedly, producing
the brassy sound heard during forced expiration. This does not occur in normal
expiration because the intrapleural pressure remains negative throughout. In
diseases in which the airways are already narrowed (e.g. asthma), this
leads to expiratory wheezing and air trapping.
Lung function tests
Lung volumes can be measured using
a simple spirometer (Chapter 25). Airway resistance and lung compliance can be
assessed indirectly by measuring the forced expiratory flows and volumes. The
easiest and quickest measurement is the peak expiratory flow rate (PEFR).
PEFR is decreased if the airway resistance is increased (obstructive disease),
and is commonly used to follow an already diagnosed condition, e.g. asthma. It
is, however, dependent on the initial lung volume (Fig. 26e). Plots of the forced
expiratory volume against time provide more information. Subjects breathe
out from total lung capacity to residual volume as fast as possible; this is
the forced vital capacity (FVC), and a typical trace is shown in Figure
26g. The forced expiratory volume in 1 s (FEV1) reflects the airway
resistance; it is normally expressed as a ratio to FVC (FEV1/FVC) to
correct for lung volume, and is usually 0.75–0.90. It can be used to
distinguish between obstructive (increased airway resistance) and restrictive
(decreased lung compliance) diseases. In asthma, for example, FEV1/FVC is typically
<0.7. In restrictive disease (e.g. lung fibrosis), FEV1 and FVC are low, but FEV1/FVC is normal or even increased due to greater elastic recoil (Fig. 26g).