Although DNA determines the type of
biochemical product needed by the cell and directs its synthesis, it is RNA through
the process of translation, which is responsible for the actual assembly of the
products.
RNA Structure and Function
RNA, like DNA, is a large molecule made
up of a long string of nucleotides. However, it differs from DNA in three
aspects of its structure. First, RNA is a single-stranded rather than a double-stranded
molecule. Second, the sugar in each nucleotide of RNA is ribose instead of deoxyribose.
Third, the pyrimidine base thymine in DNA is replaced by uracil in RNA.
Cells contain three types of RNA: messenger
RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). All three types of RNA
are synthesized in the nucleus by RNA polymerase enzymes and then moved into
the cytoplasm, where protein synthesis takes place. Messenger RNA carries
the instructions for protein synthesis, obtained from the DNA molecule, into the
cytoplasm. Transfer RNA reads the instructions and delivers the appropriate
amino acids to the ribosome, where ribosomal RNA translates the instructions
and provides the machinery needed for protein synthesis.
Messenger RNA. Messenger RNA is the template for protein
synthesis. It is a long molecule containing several hundred to several thousand
nucleotides. Each group of three nucleotides forms a codon that is exactly complementary
to a nucleotide triplet of the DNA
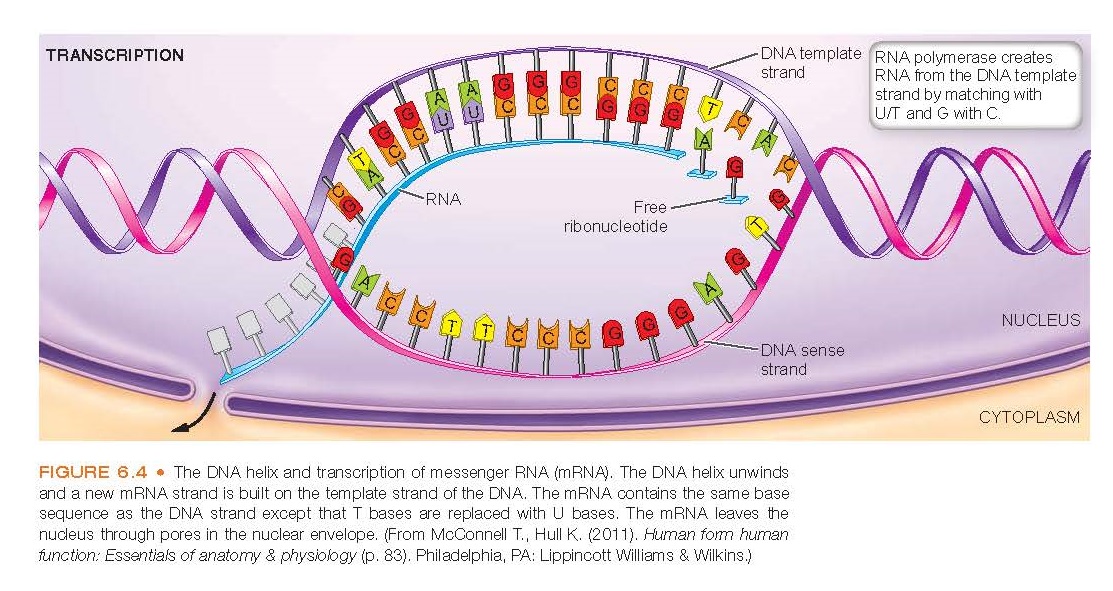
molecule. Messenger RNA is formed by a process called transcription.
In this process, the weak hydro- gen bonds of DNA are broken so that free
RNA nucleotides can pair with their exposed DNA counterparts on the meaningful strand
of the DNA molecule (see Fig. 6.4). As with the base pairing of the DNA strands,
complementary RNA bases pair with the DNA bases. In RNA, uracil (U) replaces thymine
and pairs with adenine. As with DNA, guanine pairs with cytosine.
Ribosomal RNA. The ribosome is the physical structure in the cytoplasm
where protein synthesis takes place. Ribosomal RNA forms 60% of the ribosome, with
the remainder of the ribosome composed of the structural proteins and enzymes
needed for protein synthesis. As with the other types of RNA, rRNA is synthesized
in the nucleus. Unlike the two other types of RNA, rRNA is produced in a specialized
nuclear structure called the nucleolus. The formed rRNA combines with ribosomal
proteins in the nucleus to produce the ribosome, which is then transported into
the cytoplasm. On reaching the cytoplasm, most ribosomes become attached to the
endoplasmic reticulum and begin the task of protein synthesis.
Transfer RNA. Transfer RNA is a clover-shaped molecule containing only 80 nucleotides, making it the
smallest RNA molecule. Its function is to deliver the activated form of an amino
acid to the protein that is being synthesized in the ribosomes. At least 20 different
types of tRNA are known, each of which recognizes and binds to only one type of
amino acid. Each tRNA molecule has two recognition sites: the first is complementary
for the mRNA codon and the second for the amino acid itself. Each type of tRNA carries its own specific amino acid to the ribosomes, where protein synthesis is
taking place; there it recognizes the appropriate codon on the mRNA and delivers
the amino acid to the newly forming protein molecule.
Transcription
Transcription occurs in the cell nucleus
and involves the syn- thesis of RNA from a DNA template (Fig. 6.4). Genes are
transcribed by enzymes called RNA polymerases that generate a single-stranded
RNA identical in sequence (with the exception of U in place of T) to one of the
strands of DNA. It is initiated by the assembly of a transcription complex composed
of RNA polymerase and other associated factors. This complex binds to the double-stranded
DNA at a specific site called the promoter region. Within the promoter region,
the so-called TATA box is located. The TATA box contains the crucial thy-
mine–adenine–thymine–adenine (TATA) nucleotide sequence that RNA polymerase recognizes
and binds to. This binding also requires transcription factors, a transcription
initiation site, and other proteins. Transcription continues to copy the
meaningful strand into a single strand of RNA as it travels along the length of
the gene, stopping only when it reaches a termination site with a stop codon. On
reaching the stop signal, the RNA polymerase enzyme leaves the gene and releases
the RNA strand. The RNA strand then is processed.
Processing involves the addition of
certain nucleic acids at the ends of the RNA strand and cutting and splicing of
certain internal sequences. Splicing involves the removal of stretches of RNA. Because
of the splicing process, the final mRNA sequence is different from the original
DNA template. The retained protein-coding regions of the mRNA sequences are called
exons and the regions between exons are called introns. The functions
of the introns are unknown. They are thought to be involved in the activation or deactivation of genes during various stages of development.
Splicing permits a cell to produce a
variety of mRNA molecules from a single gene. By varying the splicing segments of
the initial mRNA, different mRNA molecules are formed. For example, in a muscle
cell, the original tropomyosin mRNA is spliced in as many as 10 different ways,
yielding distinctly different protein products. This permits different proteins
to be expressed from a single gene and reduces how much DNA must be contained
in the genome.
Translation
Translation occurs in the cytoplasm
of the cell and involves the synthesis of a protein using its mRNA template. Proteins
are made from a standard set of amino acids, which are joined end to end to form
the long polypeptide chains of protein molecules. Each polypeptide chain may have
as many as 100 to more than 300 amino acids in it. Besides rRNA, translation requires
the coordinated actions of mRNA and tRNA (Fig. 6.5). Each of the 20 different
tRNA molecules transports its specific amino acid to the ribosome for incorporation
into the developing protein molecule. Messenger RNA provides the information needed
for placing the amino acids in their proper order for each specific type of protein.
During protein synthesis, mRNA contacts and passes through the ribosome, during
which it “reads” the directions for protein synthesis. As mRNA passes through
the ribosome, tRNA delivers the appropriate amino acids for attachment to the growing
polypeptide chain. The long mRNA molecule usually travels through and directs protein
synthesis in more than one ribosome at a time. After the first part of the mRNA
is read by the first ribosome, it moves onto a second and a third. As a result,
ribosomes that are actively involved in protein synthesis are often found in clusters
called polyribosomes.
The process of translation is not over
when the genetic code has been used
to create the sequence of amino acids that constitute a protein. To be useful to
a cell, this new polypeptide chain must fold up into its unique three-dimensional
conformation. The folding of many proteins is made more efficient by special classes
of proteins called molecular chaperones. Typically the function of a chaperone
is to assist a newly synthesized polypeptide chain to attain a functional conformation
as a new protein and then to assist the protein’s arrival at the site in the cell
where the protein carries out its function. Molecular chaperones also assist in
preventing the misfolding of existing proteins. Disruption of chaperoning mechanisms
causes intracellular molecules to become denatured and insoluble. These denatured
proteins tend to stick to one another, precipitate, and form inclusion bodies. The
development of inclusion bodies is a common pathologic process in Parkinson, Alzheimer,
and Huntington diseases.
A newly synthesized polypeptide chain
may also need to combine with one or more polypeptide chains from the same or an
adjacent chromosome, bind small cofactors for its activity, or undergo appropriate
enzyme modification. During the posttranslation process, two or more peptide chains
may combine to form a single product. For example, two α-globin chains and two β-globin
chains combine to form the α2β2-hemoglobin molecule. The protein
products may also be modified chemically by the addition of various types of functional
groups. For example, fatty acids may be added, providing hydrophobic regions for
attachment to cell membranes. Other modifications may involve cleavage of the protein,
either to remove a specific amino acid
sequence or to split the molecule into
smaller chains. As an example, the two chains that make up the circulating active
insulin molecule, one containing 21 and the other 30 amino acids, were originally
part of an 82-amino-acid
proinsulin molecule.