Chromosomal
Disorders
Chromosomal disorders form a major
category of genetic disease, accounting for a large proportion of reproductive
wastage (early gestational abortions), congenital malformations, and intellectual
disability. Cytogenetics is
the term given to chromosome disorders, and they are
classified using the International System for Human Cytogenetic Nomenclature.
During cell division in non–germ cells, the chromosomes replicate so that each
cell receives a full diploid number. In germ cells, a different form of
division called meiosis takes place. During meiosis, the double sets of 22
autosomes and the 2 sex chromosomes (normal diploid number) are reduced to
single sets (haploid number) in each gamete. At the time of conception, the
haploid number in the ovum and that in the sperm join and restore the diploid number of chromosomes.
Chromosomal abnormalities are
commonly described according to the shorthand description of the karyotype. In
this system, the total number of chromosomes is given first, followed by the
sex chromosome complement, and then the description of any abnormality. For
example, a male with trisomy 21 is designated 47,XY,+21.
The aberrations underlying
chromosomal disorders may take the form of alterations in the structure of one
or more chromosomes or an
abnormal number of
chromosomes. Occasionally,
mitotic errors in early development give rise to two or more cell lines characterized by distinctive karyotypes, a
condition referred to as mosaicism. Mosaicism can result from mitotic
errors during cleavage of the fertilized ovum or in somatic cells. Sometimes,
mosaicism consists of an abnormal karyotype and a normal one, in which case the
physical deformities caused by the abnormal cell line usually are less severe.
Structural Chromosomal Abnormalities Structural changes in chromosomes usually
result from breakage in one or more of the chromosomes followed by
rearrangement or deletion of the chromosome parts. Among the factors believed to cause chromosome breakage
are exposure to radiation sources, such as x-rays; influence of certain chemicals;
extreme changes in the cellular environment; and viral infections.
Several patterns of chromosome
breakage and rearrangement can occur (Fig. 7.7). There can be a deletion of
the broken portion of the chromosome. When one chromosome is involved, the
broken parts may be inverted. Isochromosome formation occurs when the
centromere, or central portion, of the chromosome separates horizontally
instead of vertically. Ring formation results when deletion is followed
by uniting of the chromatids to form a ring. Translocation occurs when
there are simultaneous breaks in two chromosomes from different pairs, with
exchange of chromosome parts. With a balanced reciprocal translocation, no
genetic information is lost; therefore, persons with translocations usually are
normal. However, these people are translocation carriers and may have normal or
abnormal children.
A special form of translocation
called a centric fusion or robertsonian translocation involves
two acrocentric chromosomes in which the centromere is near the end, most commonly
chromosomes 13 and 14 or 14 and 21. Typically, the break occurs near the
centromere affecting the short arm in one chromosome and the long arm in the
other. Transfer of the chromosome fragments leads to one long and one extremely
short fragment. The short fragment is usually lost during sub- sequent
divisions. In this case, the person has only 45 chromosomes, but the amount of
genetic material that is lost is so small that it often goes unnoticed.
Difficulty, however, arises during meiosis; the result is gametes with an unbalanced
number of chromosomes. The chief clinical importance of this type of
translocation is that carriers of a robertsonian translocation involving
chromosome 21 are at risk for producing a child with Down syndrome.
The manifestations of aberrations
in chromosome structure depend to a great extent on the amount of genetic material
that is lost or displaced. Many cells sustaining unrestored breaks are
eliminated within the next few mitoses because of deficiencies that may in
themselves be fatal. This is beneficial because it prevents the damaged cells
from becoming a permanent part of the organism or, if it occurs in the gametes,
from giving rise to grossly defective zygotes. Some altered chromosomes, such
as those that occur with translocations, are passed on to the next generation.
Numeric Disorders Involving
Autosomes
Having an abnormal number of
chromosomes is referred to as aneuploidy. Among the causes of aneuploidy is a
failure of the chromosomes to separate during oogenesis or spermatogenesis.
This can occur in either the autosomes or the sex chromosomes and is called nondisjunction
(Fig. 7.8). Nondisjunction gives rise to germ cells that have an even
number of chromosomes (22 or 24). The products of conception formed from this
even number of chromosomes have an uneven number of chromosomes, 45 or 47. Monosomy
refers to the presence of only one member of a chromosome pair. The defects
associated with monosomy of the autosomes are severe and usually cause
abortion. Monosomy of the X chromosome (45,X), or Turner syndrome, causes less
severe defects.
Polysomy, or the presence of more than two chromosomes to
a set, occurs when a germ cell containing more than 23 chromosomes is involved
in conception. Trisomy 18 (Edwards syndrome)
and trisomy 13
(Patau syndrome) share several karyotypic and clinical features
with trisomy 21 (Down syndrome). In contrast to Down syndrome, however, the
malformations are much more severe and wide-ranging. As a result, these infants
rarely survive beyond the first years of life.
Down Syndrome. First described in 1866 by John Langdon Down,
trisomy 21, or Down syndrome, causes a combination of birth defects including
some degree of intellectual disability, characteristic facial features, and
other health problems. It is the most common chromosomal disorder.
Approximately 95% of cases of Down
syndrome are caused by nondisjunction or an error in cell division during
meiosis, resulting in a trisomy of chromosome 21. A rare form of Down syndrome
can occur in the offspring of people in whom there has been a robertsonian
translocation (see Fig. 7.7) involving the long arm of chromosome 21q and the
long arm of one of the acrocentric chromosomes (most often 14 or 22). The
translocation adds to the normal long arm of chromosome 21. Therefore, the person with this type of
Down syndrome has 46 chromosomes, but essentially a trisomy of 21q.
The risk of having a child with
Down syndrome increases with maternal age. The reason for the correlation
between maternal age and nondisjunction is unknown, but is thought to reflect
some aspect of aging of the oocyte. Although men continue to produce sperm
throughout their reproductive life, women are born with all the oocytes they
ever will have. These oocytes may change as a result of the aging process. With
increasing age, there is a greater chance of a woman having been exposed to
damaging environmental agents such as drugs, chemicals, and radiation. Unlike
trisomy 21, Down syndrome due to a chromosome (21;14) translocation shows no
relation to maternal age but has a relatively high recurrence risk in families
when a parent, particularly the mother, is a carrier.
A child with Down syndrome has
specific physical characteristics that are classically evident at birth. These features
include a small and rather square head. There is a flat facial profile, with a
small nose and somewhat depressed nasal bridge; small folds on the inner
corners of the eyes (epicanthal folds) and upward slanting of the eyes; small,
low-set, and malformed ears; a fat pad at the back of the neck; an open mouth;
and a large, protruding tongue (Fig. 7.9). The child’s hands usually are short
and stubby, with fingers that curl inward, and there usually is only a single
palmar (i.e., simian) crease. There is excessive space between the large
and second toes. Hypotonia and joint laxity also are present in infants and
young children. There often are accompanying congenital heart defects and an
increased risk of gastrointestinal malformations. Approximately 1% of people
with trisomy 21 Down syndrome have mosaicism (i.e., cell populations
with the normal chromosome number and trisomy 21). These people may be less
severely affected. There is a high correlation of the development of acute
leukemia, both myeloid and lymphoblastic, among children with Down
syndrome. In addition, there is an increased risk of Alzheimer disease
among older people with Down
syndrome, and many of these children have a higher chance of acquiring cardiovascular disease.
There are several prenatal
screening tests that can be done to determine the risk of having a child with
Down syndrome.18 The most commonly used are blood tests that measure maternal
serum levels of α-fetoprotein (AFP), human chorionic gonadotropin (hCG),
unconjugated estriol, inhibin A, and pregnancy-associated plasma protein A
(PAPP-A) (see section on Diagnosis and Counseling). The results of three or
four of these tests, together with the woman’s age, often are used to determine
the probability of a pregnant woman having a child with Down syndrome. Nuchal
translucency (sonolucent space on the back of the fetal neck) is another test
that can be done to assess this aspect of the fetus by uses ultrasonography and
can be performed between 10 and 13 weeks’ gestation. The fetus with Down
syndrome tends to have a greater area of translucency compared with a
chromosomally normal infant. The nuchal transparency test is usually used in
combination with other screening tests. The only way to accurately determine
the presence of Down syndrome in the fetus is through chromosome analysis using
chorionic villus sampling, amniocentesis, or percutaneous umbilical blood
sampling, which is discussed later
in this chapter.
Numeric Disorders Involving
Sex Chromosomes
Chromosomal disorders associated
with the sex chromosomes are much more common than those related to the
autosomes, except for trisomy 21. Furthermore, imbalances (excess or deletions)
are much better tolerated than those involving the autosomes. This is related
in a large part to two factors that are peculiar to the sex chromosomes:
• The
inactivation of all but one X chromosome
• The modest
amount of genetic material that is carried on the Y chromosome
Although girls normally receive
both a paternal and a maternal X chromosome, the clinical manifestations of X
chromosome abnormalities can be quite variable because of the process of X
inactivation (previously discussed in Chapter 6). In somatic cells of females,
only one X chromosome is transcriptionally active. The other chromosome is
inactive. The process of X inactivation, which is random, occurs early in
embryonic life and is usually complete at about the end of the first week of
development. After one X chromosome has become inactivated in a cell, all cells
descended from that cell have the same inactivated X chromosome. Although much
of one X chromo- some is inactivated in females, several regions contain genes
that escape inactivation and continue to be expressed by both X chromosomes.
These genes may explain some of the variations in clinical symptoms seen in
cases of numeric abnormalities of the X chromosome, such as Turner syndrome.
It is well known that the Y
chromosome determines the male sex. The gene that dictates testicular
development (Sry: sex-determining region Y gene) has been located on its
distal short arm. Recent studies of the Y chromosome have yielded additional
information about gene families in the so-called “male-specific Y” or MSY region. All of these are believed to be involved in spermatogenesis. A few
additional genes with homologs on the X chromosome have been mapped to the Y
chromosome, but to date, no disorders resulting from mutations in these genes
have been described.
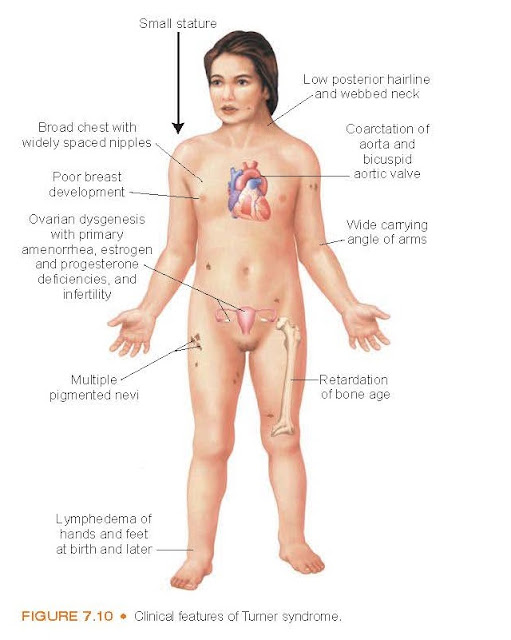
Turner Syndrome. Turner syndrome describes an absence of all
(45,X/0) or part of the X chromosome. Some women with Turner syndrome may have
part of the X chromosome, and some may display a mosaicism with one or more
additional cells lines. This disorder affects approximately 1 of every 2500
live births and is the most frequent occurring genetic disorder in women.
Characteristically, the girl with
Turner syndrome is short in stature, but her body proportions are normal (Fig.
7.10). Females with Tuner syndrome lose the majority of their oocytes by the
age of 2 years. Therefore, they do not menstruate and shows no signs of
secondary sex characteristics. There are variations in the syndrome, with
abnormalities ranging from essentially none to cardiac abnormalities such as
bicuspid aortic valve and coarctation of the aorta, problems with hearing and
vision, a small size mandible, a horseshoe kidney, and a small webbed neck.
Women with Turner syndrome have been found to develop autoimmune disorders
associated with male predominance, such as type 1 diabetes mellitus and Hashimoto thyroiditis.
Although most women with Turner
syndrome have normal intelligence, they may have problems with visuospatial
organization (e.g., difficulty in driving, nonverbal problem-solving
tasks such as mathematics, and psychomotor skills) and attention deficit
disorders.
The diagnosis of Turner syndrome
often is delayed until late childhood or early adolescence in girls who do not present
with the classic features of the syndrome. Only about 20% to 33% of affected
girls receive a diagnosis as a new-born because of puffy hands and feet or
redundant nuchal skin. Another 33% are diagnosed in mid-childhood because of
short stature. The remainder of the girls are mainly diagnosed in adolescence
when they fail to enter puberty. It is important to diagnose girls with Turner
syndrome as early as possible so treatment plans could be implemented and managed
throughout their lives.
The management of Turner syndrome
begins during childhood and requires ongoing assessment and treatment. Growth
hormone therapy generally can result in a gain of 6 to 10 cm in final height.
Estrogen therapy, which is instituted around the normal age of puberty, is used
to promote development and maintenance of secondary sexual characteristics.
Klinefelter Syndrome. Klinefelter syndrome is a condition of
testicular dysgenesis accompanied by the presence of one or more extra X
chromosomes in excess of the normal male XY complement. Most males with
Klinefelter syndrome have one extra X chromosome (47,XXY). In rare cases, there
may be more than one extra X chromosome (48,XXXY). The presence of the extra X
chromosome in the 47,XXY male results from nondisjunction during meiotic
division in one of the parents. The extra X chromosome is usually of maternal
origin, but approximately 1/3 of the time, it is of paternal origin. The cause
of the nondisjunction is unknown. Advanced maternal age increases the risk, but
only slightly. Klinefelter syndrome occurs in approximately 1 per 1000 newborn
male infants.
Although the presence of the extra
chromosome is fairly common, the syndrome with its accompanying signs and
symptoms that may result from the extra chromosome is uncommon. Many men live
their lives without being aware that they have an additional chromosome. For
this reason, it has been suggested that the term Klinefelter syndrome be
replaced with 47,XXY male.
Klinefelter syndrome is
characterized by enlarged breasts, sparse facial and body hair, small testes,
and the inability to produce sperm (Fig. 7.11). Regardless of the number of X
chromosomes present, the male phenotype is retained. The condition often goes
undetected at birth. The infant usually has normal male genitalia, with a small
penis and small, firm testicles. At puberty, the intrinsically abnormal testes
do not respond to stimulation from the gonadotropins and undergo degeneration.
This leads to a tall stature with abnormal body proportions in which the lower
part of the body is longer than the upper part. Later in life, the body build
may become heavy, with a female
distribution of subcutaneous fat and variable degrees of breast enlargement. There may be deficient secondary male sex
characteristics, such as a voice that remains feminine in pitch and sparse
beard and pubic hair. Although the intellect usually is normal, most 47,XXY
males have some degree of language impairment.
Adequate management of Klinefelter
syndrome requires a comprehensive neurodevelopmental evaluation. In infancy and
early childhood, this often includes a multidisciplinary approach to determine
appropriate treatments such as physical therapy, infant stimulation programs,
and speech therapy. Men with Klinefelter syndrome have congenital hypogonadism,
which results in an inability to produce normal amounts of testosterone accompanied
by an increase in hypothalamic gonadotrophic hormones. Androgen therapy is
usually initiated when there is evidence of a testosterone deficit. Infertility
is common in men with Klinefelter syndrome because of a decreased sperm count.
If sperm are present, cryopreservation may
be useful for future family planning. However, genetic counseling is advised because of the increased risk
of autosomal and sex chromosomal abnormalities. Men with Klinefelter syndrome
also experience increased risk for osteoporosis and need to be educated on
prevention management.