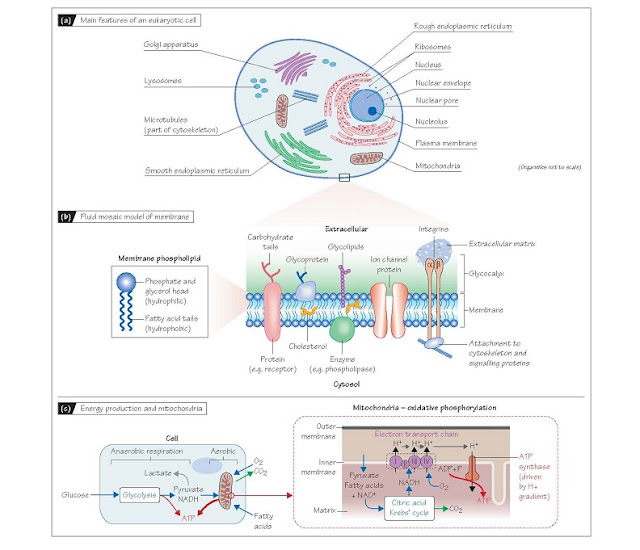
Cells, Membranes And Organelles
The aqueous internal environment of the cell is separated from the
aqueous external medium by an envelope of fat molecules (lipids) known
as the plasma membrane. About half the cell is filled with cytosol,
a viscous, protein-rich fluid between the internal structures. These consist of
organelles which are themselves enclosed by lipid membranes, and
components of the cytoskeleton such as microtubules and actin filaments
which provide structural stability. The reticular appearance of the cell
interior is due to organelles whose membranes are folded to maximize surface
area. These include the rough endo- plasmic reticulum and Golgi
apparatus, which are involved in protein assembly, and the smooth
endoplasmic reticulum which serves as a store for intracellular Ca2+ and is
the major site of lipid production (Fig. 3a).
Protein-processing organelles
The nucleus (Fig. 3a)
contains the chromosomes and nucleolus, a membrane-less structure
responsible for production of ribosomes. Ribosomes translocate to the rough
endoplasmic reticulum (giving it its appearance), where they are
responsible for protein assembly. The endoplasmic reticulum and Golgi
apparatus perform post-trans- lational processing of new proteins. This
includes trimming amino acid chains to the right length, protein folding,
addition of polysac- charide chains (glycosylation) and identification
of improperly folded proteins, which are tagged for subsequent destruction by
lysosomes. Proteins are delivered from the Golgi apparatus to specific
intracellular destinations. For example, receptor and structural proteins are
sent to the membrane and digestive enzymes to lysosomes, and molecules for
extracellular action are packaged into secretory vesicles. Lysosomes contain
acid hydrolase enzymes which catabolize macromolecules.
They work optimally at pH 5.0, and
as cytosolic pH is ∼7.2, anyleaking into the cytosol cannot attack
the cell inappropriately. Lysosomes digest unwanted and defective proteins,
recycling raw materials and preventing accumulation of rubbish.
Membranes and
membrane proteins Membrane lipids (mostly
phospholipids) comprise a hydrophilic
(water-loving) head, with two short hydrophobic (water-repelling)
fatty acid tails (Fig. 3b). In an aqueous medium they self-organize into a bilayer
with the heads facing outwards and the tails inwards (Fig. 3b). They
diffuse freely within each layer (lateral diffusion) so the membrane is
fluid. The hydrophobic interior and hydrophilic exte- rior of the membrane
means that lipid-soluble (hydrophobic) sub- stances such as cholesterol incorporate
into the membrane, whilst molecules with both hydrophobic and hydrophilic
domains such as proteins can be tethered part in and part out of the membrane
(the fluid mosaic model; Fig 3b). Many such molecules provide
signalling, transport or structural functions. The latter are provided by
proteins such as spectrin, which binds to the inner layer and forms an attachment
framework for the cytoskeleton. Lipid-soluble molecules such as O2
and CO2, and small molecules such as water and urea readily pass
through the lipid bilayer. However, larger molecules such as glucose and polar
(charged) molecules such as ions cannot, and their transport is mediated by transporter
and ion channel membrane proteins (Chapter 4). Membrane proteins
also undergo lateral diffusion and move around the membrane. However, the cell
can control exactly which proteins
insert into which portion of the membrane. For example, cells lining the kidney
tubules are polarized so that Na+ –K+ ATPase transporters (Chapters 4
and 33) are located only on one side of the cell. Most cells are covered by a
thin gel-like layer called the glycocalyx, containing glycoproteins and
carbohydrate chains extending from the membrane and secreted proteins (Fig.
3b). It protects the membrane and also plays a role in cell function and
cell–cell interactions.
Membrane proteins associated with
cell signalling include enzymes bound to the inner surface such as phospholipases,
which produce arachidonic acid (a precursor of some second messenger
molecules), and adenylyl cyclase, which generates the second messenger
cyclic adenosine monophosphate (cAMP). cAMP activates protein kinase enzymes
to initiate numerous changes in cell function by phosphor- ylating membrane
and intracellular proteins. Transmembrane proteins (Fig. 3b) penetrate
the entire thickness of the bilayer, and include receptors and ion
channel proteins. The intramembrane segments are composed of hydrophobic
amino acid residues and the extra- and intra-cellular portions predominantly of
hydrophilic residues. Receptors include those that bind growth factors and
regulate gene transcription, and the superfamily known as G-protein–coupled
receptors (GPCRs). The latter possess seven membrane-spanning segments and
detect neurotransmitters or hormones in the extracellular medium. On binding
the appropriate molecule, they activate specific mem- brane-associated GTP-binding
proteins (G-proteins), which cleave guanosine triphosphate (GTP) to
guanosine diphosphate (GDP), and depending on type (e.g. Gs, Gi, Gq), activate
or inhibit other membranebound signalling enzymes such as adenylyl cyclase.
Transmembrane proteins such as integrins and cadherins provide
structural and signalling links with other cells and the extracellular
matrix (Fig. 3b). Their cytosolic ends bind to components of the
cytoskeleton, including protein kinases which can initiate, for example, altered
gene transcription or changes in cell shape.
Mitochondria and
energy production Mitochondria use molecular oxygen to, in effect, burn sugar
and small fatty acid molecules to produce adenosine triphosphate (ATP),
which is used by all energy-requiring cellular reactions. Glucose is first
converted to pyruvate in the cytosol by glycolysis, producing in the process a
small net amount of ATP and reduced nicotinic adenine dinucleotide (NADH).
Glycolysis does not require O2, so when O2 is limited, this anaerobic respiration can
supply some ATP, with NADH being reoxidized to NAD+ by metabolism of the pyruvate to lactate
(Fig. 3c). However, under normal conditions where there is sufficient O2,
oxidative phosphorylation in the mitochondria produces ∼15 fold
more ATP for each glucose molecule than does glycolysis. Pyruvate and fatty
acids transported into the mitochondrial matrix act as substrates for
enzymes that drive the citric acid (Krebs’) cycle, which generates NADH
and the waste product CO2. The electron transport chain,
a series of enzymes in the inner mitochondrial membrane, then uses molecular O2
to re-oxidize NADH to NAD+. In doing so, it generates a H+ ion gradient across the inner membrane which
drives the ATP synthase (Fig. 3c). Note that mitochondria are not solely
devoted to ATP production, as they are also involved in other cellular
processes, including Ca2+ homeostasis and signalling.