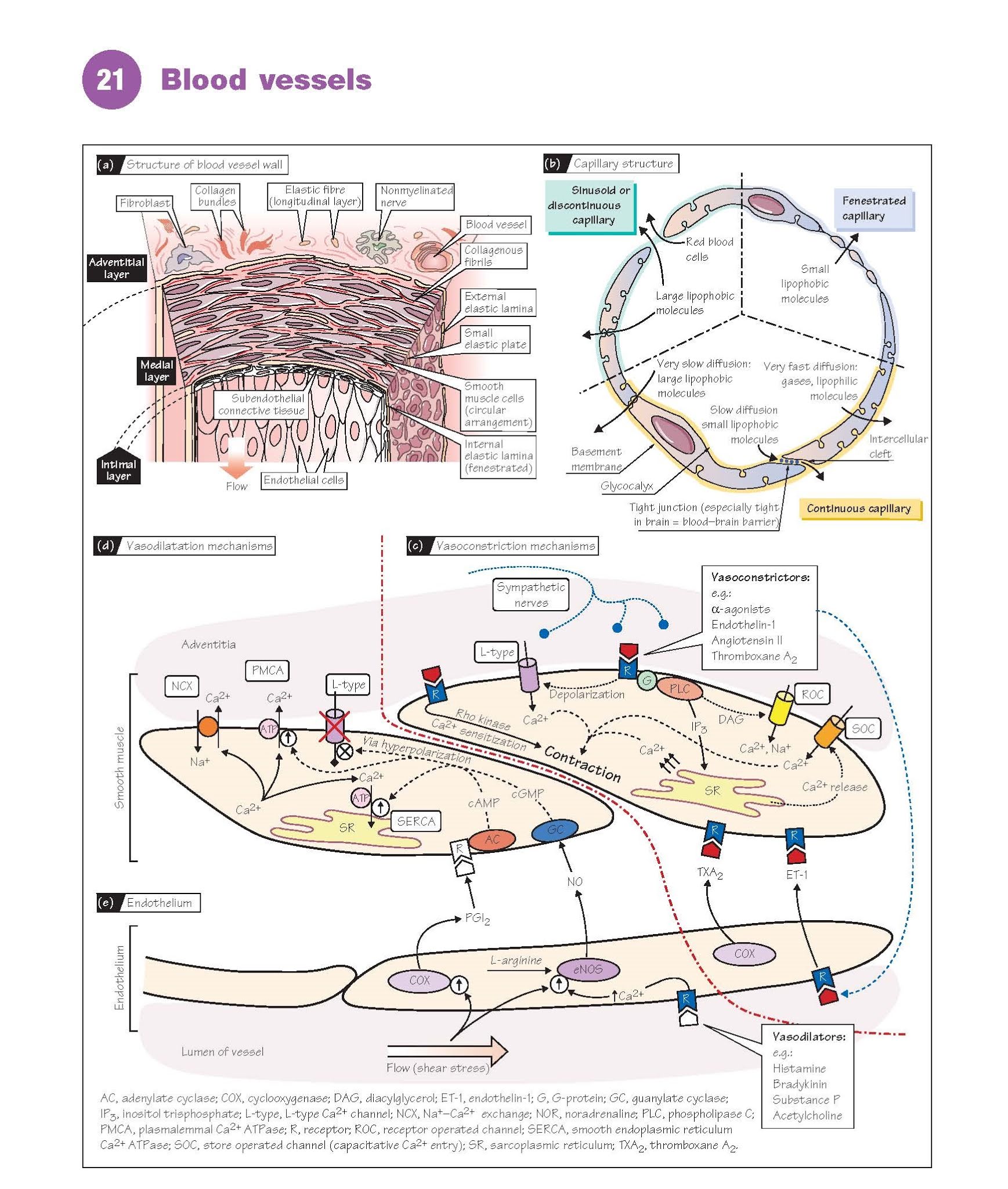
Blood Vessels
Structure
The walls of larger blood vessels
comprise three layers: an inner intima (tunica intima) consisting
of a thin layer of endothelial cells; a thick media (tunica
media) containing smooth muscle and elastin filaments that
provide elastic properties; and an outer adventitia (tunica
adventitia) consisting of fibroblasts and nerves embedded in collagenous
tissue (Fig. 21a). The layers are separated by inner and outer elastic
lamina. In large vessels, the adventitia contains a network of blood vessels
called the vasa vasorum (vessel of vessels) supplying the smooth
muscle. Veins have a thinner media than arteries, and contain less smooth
muscle. All three layers contain fibrous collagen, which acts as a
framework to which cells are anchored.
Vascular smooth muscle cells are elongated, 15–100 μm in length, and
tend to be orientated in a spiral around the vessel; the lumen therefore
narrows as they contract. Cells are connected by gap junctions,
allowing electrical coupling and depolarization to spread from cell to cell.
The structure and function of smooth muscle are described in Chapter 15.
Capillaries and the smallest venules are formed from a
single layer of endothelial cells supported on the outside by a 50–100-nm thick
basal lamina containing collagen. The luminal surface is covered by a glycoprotein
network called the glycocalyx. There are three basic types of capillary,
varying in permeability (Fig. 21b). Continuous capillaries have a low
permeability, as junctions between the endothelial cells are very tight and
prevent the diffusion of lipophobic molecules of >10 000 Da. They are found
in skin, lungs, central nervous system and muscle. Fenestrated capillaries have
less tight junctions and the endothelial cells are also punctured by 50–100-nm
pores (fenestrae); they are therefore much more permeable. They are
found where large amounts of fluid or material need to diffuse across the
capillary wall, including endocrine glands, renal glomeruli and intestinal
villa. Discontinuous capillaries are found in bone marrow, liver and
spleen, and have gaps large enough for red blood cells to pass through. The
microcirculation is discussed further in Chapter 23.
Regulation of function and
excitation–contraction coupling
Vasoconstriction (Fig. 21c). Most vasoconstrictors bind to
receptors and cause a guanosine triphosphate-binding protein (G-protein)-mediated elevation in intracellular [Ca2+], leading to contraction. Important
vasoconstrictors include endothelin-1, angiotensin II (Chapter 35) and the
sympathetic transmitter noradrenaline (norepine- phrine) (Chapter 7).
Ca2+ release.
Binding to a receptor activates phospholipase C, which generates the second messengers inositol
trisphosphate (IP3) and diacylglycerol (DAG) from membrane
phospholipids. IP3 binds to receptors on the sarcoplasmic reticulum (SR)
causing Ca2+ channels to open and Ca2+ to flood into the cytoplasm. This
response may only be transient as the store rapidly empties, but may initiate capacitative
Ca2+ entry (see below).
Ca2+ entry. Vasoconstrictors also cause
depolarization, which activates Ca2+ entry via L-type voltage-gated Ca2+
channels as in cardiac muscle (Chapter 19). Unlike cardiac muscle, most
types of vascular smooth muscle do not generate action potentials, but instead
depolari- zation is graded, allowing graded entry of Ca2+. Receptor operated
channels (ROC) may also be activated, some by
DAG, through which both Ca2+ and
Na+ can enter the cell; the latter contributes to depolarization.
IP3-stimulated emptying of Ca2+ stores can also directly activate store
operated channels (SOC) in the membrane, causing capacitative Ca2+ entry.
Importantly, many agonists also
cause Ca2+ sensitization of the contractile apparatus, i.e. more
force for the same rise in Ca2+. This is mediated by Rho kinase,
although protein kinase C, which is activated by DAG, may also be involved. The
relative importance of the above mechanisms depends on the vascular bed and
vasoconstrictor. In small-resistance arteries, depolarization and voltage-gated
Ca2+ entry are probably most important. Most systemic arteries exhibit a degree
of basal (myogenic) tone in the absence of
vasoconstrictors.
Ca2+ removal and vasodilatation (Fig. 21d).
Ca2+ is pumped back into the SR (sequestrated) by the smooth
endoplasmic reticulum Ca2+ ATPase (SERCA) which can rapidly reduce
cytosolic Ca2+. Ca2+ is also removed from the cell by a plasma membrane Ca2+
ATPase (PMCA) and Na+–Ca2+ exchange (NCX; Chapter 19). Most
endogenous vasodilators cause relaxation by increasing cyclic guanosine
monophosphate (cGMP) (e.g. nitric oxide, NO) or cyclic adenosine
monophosphate (cAMP) (e.g. prostacyclin, β-adrenergic receptor
agonists). These second messengers act via protein kinase G (PKG) or protein
kinase A (PKA), respectively. Both PKG and PKA lower intracellular Ca2+, partly
by stimulating SERCA and PMCA, and partly by hyperpolarizing the membrane (i.e.
so voltage-gated Ca2+ entry is inhibited). L-type Ca2+ channel blocker drugs,
such as verapamil or dihydropyridines, are clinically effective
vasodilators.
The endothelium (Fig. 21e)
The endothelium plays a crucial
role in the regulation of vascular tone. In response to substances in the blood
or changes in blood flow, it can synthesize several important vasodilators,
including NO (endothelium-derived relaxing factor, EDRF) and prostacyclin
(prostaglandin I2, PGI2), as well as potent vasoconstrictors, such as endothelin-1
and thromboxane A2 (TXA2).
NO is synthesized by the endothelial nitric
oxide synthase (eNOS) from
l-arginine. eNOS activity and NO production are increased by factors that
elevate intracellular Ca2+, including local mediators such as bradykinin,
histamine and serotonin, and some neurotransmitters (e.g. substance P). Increased flow (shear stress)
also stimulates NO production,
and additionally activates prostacyclin synthesis. The basal production of NO
continuously modulates vascular resistance, as it has been found that
inhibition of eNOS causes the blood pressure to rise. NO also inhibits platelet
activation and thrombosis (inappropriate clotting) (Chapter 9).
Endothelin-1 is an extremely potent vasoconstrictor peptide
which is released from the endothelium in the presence of many other vasoconstrictors,
including angiotensin II, antidiuretic hormone (ADH; vasopressin) and
noradrenaline, and may be increased in disease and hypoxia. As endothelin
receptor blockade causes a fall in the peripheral resistance of healthy humans,
it seems to contribute to the maintenance of blood pressure.
The eicosanoids prostacyclin
and TXA2 are synthesized by the cyclooxygenase pathway from arachidonic acid,
which is made from membrane phospholipids by phospholipase A2. In most vessels prostacyclin
is most important.